Brand Name
Viread
Generic Name
Tenofovir Disoproxil
View Brand Information FDA approval date: October 26, 2001
Classification: Human Immunodeficiency Virus Nucleoside Analog Reverse Transcriptase Inhibitor
Form: Tablet, Powder
What is Viread (Tenofovir Disoproxil)?
Tenofovir disoproxil fumarate tablets are a nucleotide analog HIV-1 reverse transcriptase inhibitor and an HBV reverse transcriptase inhibitor. Tenofovir disoproxil fumarate tablets are indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 2 years of age and older., Tenofovir disoproxil fumarate tablets are indicated for the treatment of chronic hepatitis B in adults and pediatric patients 12 years of age and older.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Viread (TENOFOVIR DISOPROXIL FUMARATE)
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including VIREAD. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in HBV-infected patients who discontinue anti-hepatitis B therapy, including VIREAD. If appropriate, resumption of anti-hepatitis B therapy may be warranted [see .
1DOSAGE FORMS AND STRENGTHS
VIREAD is available as tablets in four dose strengths or as an oral powder.
- 150 mg Tablets: 150 mg of tenofovir disoproxil fumarate (TDF) (equivalent to 123 mg of tenofovir disoproxil): triangle shaped, white, film coated, debossed with "GSI" on one side and with "150" on the other side.
- 200 mg Tablets: 200 mg of TDF (equivalent to 163 mg of tenofovir disoproxil): round shaped, white, film coated, debossed with "GSI" on one side and with "200" on the other side.
- 250 mg Tablets: 250 mg of TDF (equivalent to 204 mg of tenofovir disoproxil): capsule shaped, white, film coated, debossed with "GSI" on one side and with "250" on the other side.
- 300 mg Tablets: 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil): almond shaped, light blue, film coated, debossed with "GILEAD" and "4331" on one side and with "300" on the other side.
- Oral Powder: white, taste-masked, coated granules containing 40 mg of TDF (equivalent to 33 mg of tenofovir disoproxil) per level scoop. Each level scoop contains 1 gram of oral powder.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection
- New Onset or Worsening Renal Impairment
- Immune Reconstitution Syndrome
- Bone Loss and Mineralization Defects
- Lactic Acidosis/Severe Hepatomegaly with Steatosis
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VIREAD. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders
allergic reaction, including angioedema
allergic reaction, including angioedema
Metabolism and Nutrition Disorders
lactic acidosis, hypokalemia, hypophosphatemia
lactic acidosis, hypokalemia, hypophosphatemia
Respiratory, Thoracic, and Mediastinal Disorders
dyspnea
dyspnea
Gastrointestinal Disorders
pancreatitis, increased amylase, abdominal pain
pancreatitis, increased amylase, abdominal pain
Hepatobiliary Disorders
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT gamma GT)
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT gamma GT)
Skin and Subcutaneous Tissue Disorders
rash
rash
Musculoskeletal and Connective Tissue Disorders
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
Renal and Urinary Disorders
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
General Disorders and Administration Site Conditions
asthenia
asthenia
The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
4OVERDOSAGE
If overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of VIREAD, a four-hour hemodialysis session removed approximately 10% of the administered tenofovir dose.
5DESCRIPTION
VIREAD is the brand name for tenofovir disoproxil fumarate (TDF) (a prodrug of tenofovir) which is a fumaric acid salt of bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. TDF is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. Tenofovir exhibits activity against HIV-1 reverse transcriptase.
The chemical name of TDF is 9-[(
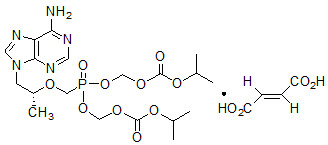
Tenofovir disoproxil fumarate is a white to off-white crystalline powder with a solubility of 13.4 mg/mL in distilled water at 25 °C. It has an octanol/phosphate buffer (pH 6.5) partition coefficient (log p) of 1.25 at 25 °C.
VIREAD is available as tablets or as an oral powder.
VIREAD tablets are for oral administration and are available in the following strengths: 150 mg, 200 mg, 250 mg, and 300 mg of TDF (equivalent to 123 mg, 163 mg, 204 mg, and 245 mg of tenofovir disoproxil, respectively).
All strengths of VIREAD tablets contain the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The 300 mg strength tablets are coated with Opadry II Y-30-10671-A, which contains FD&C blue #2 aluminum lake, hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin. The 150 mg, 200 mg, and 250 mg strength tablets are coated with Opadry II 32K-18425, which contains hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin.
VIREAD oral powder is available for oral administration as white, taste-masked, coated granules containing 40 mg of TDF per gram of oral powder (equivalent to 33 mg of tenofovir disoproxil). The oral powder contains the following inactive ingredients: mannitol, hydroxypropyl cellulose, ethylcellulose, and silicon dioxide.
In this insert, all dosages are expressed in terms of TDF except where otherwise noted.
6HOW SUPPLIED/STORAGE AND HANDLING
VIREAD tablets are available in bottles containing 30 tablets with child-resistant closure as follows:
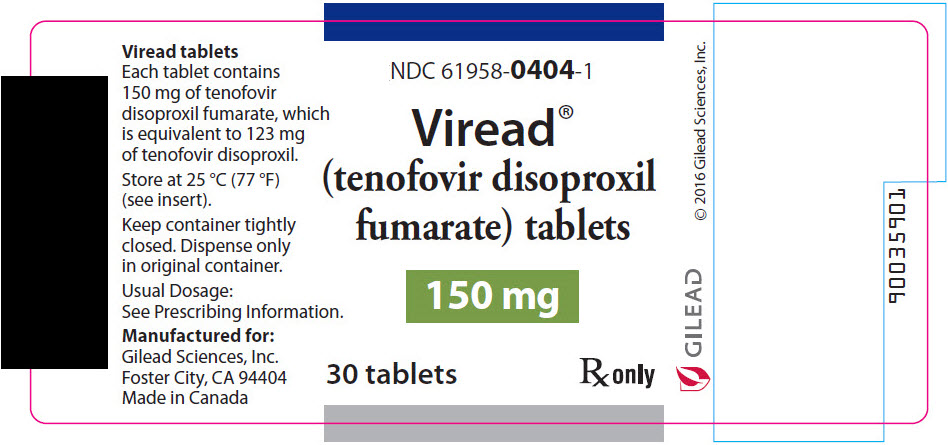
- 150 mg of TDF (equivalent to 123 mg of tenofovir disoproxil): tablets are triangle-shaped, white, film-coated, and debossed with "GSI" on one side and with "150" on the other side. (NDC 61958-0404-1)
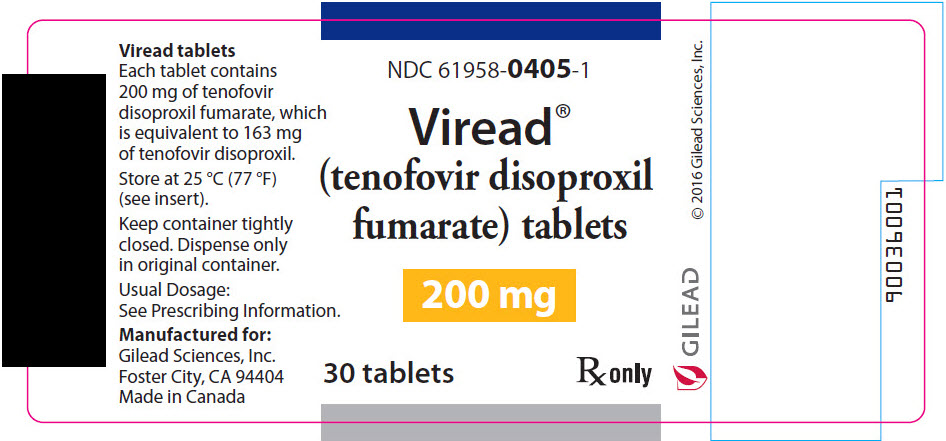
- 200 mg of TDF (equivalent to 163 mg of tenofovir disoproxil): tablets are round-shaped, white, film-coated, and debossed with "GSI" on one side and with "200" on the other side. (NDC 61958-0405-1)
- 250 mg of TDF (equivalent to 204 mg of tenofovir disoproxil): tablets are capsule-shaped, white, film-coated and debossed with "GSI" on one side and with "250" on the other side. (NDC 61958-0406-1)
- 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil): tablets are almond-shaped, light-blue, film-coated, and debossed with "GILEAD" and "4331" on one side and with "300" on the other side. (NDC 61958-0401-1)
VIREAD oral powder consists of white, coated granules containing 40 mg of TDF (equivalent to 33 mg of tenofovir disoproxil) per gram of powder and is available in multi-use bottles containing 60 grams of oral powder, closed with a child-resistant closure, and co-packaged with a dosing scoop. (NDC 61958-0403-1)
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
8Instructions for Use
VIREAD
Read the Patient Information that comes with VIREAD powder for important information about VIREAD.
Read this Instructions for Use before you give VIREAD for the first time. Be sure you understand and follow the instructions. If you have any questions, ask your healthcare provider or pharmacist.
Important information
- VIREAD powder comes in a box that has a bottle of VIREAD and a dosing scoop (see
- Only use the dosing scoop to measure VIREAD powder.
- Only mix VIREAD powder with soft foods that can be swallowed without chewing. Examples of soft foods you can use are: applesauce, baby food, or yogurt.
- Do not mix VIREAD powder with liquid. The powder may float to the top even after stirring.
- Give the entire dose right away after mixing to avoid a bad taste.
How do I prepare and give a dose of VIREAD powder?
- Wash your hands well with soap and water, and dry them.
- Measure ¼ to ½ cup of soft food such as applesauce, baby food, or yogurt into a cup or bowl.
- To open a new bottle of powder, press down on the bottle lid and turn to remove (see picture on the top of the bottle cap). Peel off the foil.
- Measure the number of scoops prescribed by your healthcare provider.
- Sprinkle the VIREAD powder on the soft food. Stir with a spoon until well mixed.
- Close the bottle of VIREAD tightly.
- Wash and dry the dosing scoop.
How should I store VIREAD powder?
- Store VIREAD powder at room temperature between 68 °F to 77 °F (20 °C to 25 °C).
- Keep VIREAD powder in the original container.
- Keep the bottle tightly closed.
- Do not use VIREAD powder if the seal over the bottle opening is broken or missing.
Keep VIREAD and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
9PRINICPAL DISPLAY PANEL - 150 mg Tablet Bottle Label
NDC 61958-0404-1
Viread
150 mg
30 tablets
R
10PRINICPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
NDC 61958-0405-1
Viread
200 mg
30 tablets
R
11PRINICPAL DISPLAY PANEL - 250 mg Tablet Bottle Label
NDC 61958-0406-1
Viread
250 mg
30 tablets
R

12PRINICPAL DISPLAY PANEL - 300 mg Tablet Bottle Label
NDC 61958-0401-1
Viread
300 mg
30 tablets
R

13PRINICPAL DISPLAY PANEL - 40 mg/Scoop Bottle Label
NDC 61958-0403-1
Viread
60 g per bottle
R
