Generic Name
Paliperidone
Brand Names
Erzofri, Invega HAFYERA, Invega, Invega SUSTENNA, Invega TRINZA
FDA approval date: December 19, 2006
Classification: Atypical Antipsychotic
Form: Injection, Tablet
What is Erzofri (Paliperidone)?
Paliperidone Extended-Release Tablets are an atypical antipsychotic agent indicated for Treatment of schizophrenia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Erzofri extended-release (paliperidone palmitate)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA- RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ERZOFRI is not approved for use in patients with dementia-related psychosis. [see .
1INDICATIONS AND USAGE
ERZOFRI is indicated for the treatment of:
- Schizophrenia in adults
- Schizoaffective disorder in adults as monotherapy and as an adjunct to mood stabilizers or antidepressants
2DOSAGE FORMS AND STRENGTHS
Extended-release injectable suspension: white to off-white aqueous suspension available in dose strengths of 39 mg/0.25 mL, 78 mg/0.5 mL, 117 mg/0.75 mL, 156 mg/mL, 234 mg/1.5 mL, and 351 mg/2.25 mL paliperidone palmitate.
Each strength is provided as a kit, which includes: one single-dose prefilled syringe and 2 safety needles (a 1 ½-inch 22 gauge needle and a 1-inch 23 gauge needle).
3CONTRAINDICATIONS
ERZOFRI is contraindicated in patients with a known hypersensitivity to either paliperidone or risperidone, or to any of the excipients in the ERZOFRI formulation. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone and in patients treated with paliperidone
4ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Increased mortality in elderly patients with dementia-related psychosis
- Cerebrovascular adverse reactions, including stroke, in elderly patients with dementia-related psychosis
- Neuroleptic malignant syndrome
- QT prolongation
- Tardive dyskinesia
- Metabolic changes
- Orthostatic hypotension and syncope
- Falls
- Leukopenia, neutropenia, and agranulocytosis
- Hyperprolactinemia
- Potential for cognitive and motor impairment
- Seizures
- Dysphagia
- Priapism
- Disruption of body temperature regulation
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of ERZOFRI for the treatment of schizophrenia in adults and schizoaffective disorder in adults as monotherapy and as an adjunct to mood stabilizers or antidepressants is based upon adequate and well-controlled studies of another once-a-month paliperidone palmitate extended-release injectable suspension (also referred to as "PP1M" in this section). Below is a display of adverse reactions with another PP1M from those adequate and well-controlled studies.
Injection site reactions for ERZOFRI presented in this section (see "
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of paliperidone; because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: angioedema, catatonia, ileus, somnambulism, swollen tongue, thrombotic thrombocytopenic purpura, urinary incontinence, and urinary retention.
Cases of anaphylactic reaction after injection with another once-a-month paliperidone palmitate extended-release injectable suspension product have been reported during postmarketing experience in patients who have previously tolerated oral risperidone or oral paliperidone.
Paliperidone is the major active metabolite of risperidone. Adverse reactions reported with oral risperidone and risperidone long-acting injection can be found in the
5DESCRIPTION
ERZOFRI (paliperidone palmitate) extended-release injectable suspension contains a racemic mixture of (+)- and (-)- paliperidone palmitate. Paliperidone palmitate, is an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. The chemical name is (9
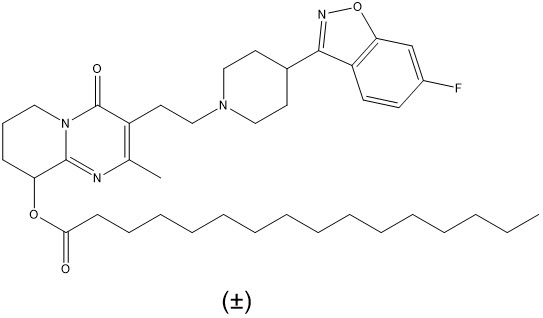
Paliperidone palmitate is very slightly soluble in ethanol and methanol, practically insoluble in water, and slightly soluble in ethyl acetate.
ERZOFRI is available as a white to off-white sterile aqueous extended-release suspension for intramuscular injection in the following dose strengths of paliperidone palmitate (deliverable volume) in single-dose prefilled syringes: 39 mg (0.25 mL), 78 mg (0.5 mL), 117 mg (0.75 mL), 156 mg (1 mL), 234 mg (1.5 mL), and 351 mg (2.25 mL). The drug product hydrolyzes in vivo to the active moiety, paliperidone, resulting in dose strengths of 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, and 225 mg of paliperidone, respectively.
The inactive ingredients are citric acid monohydrate (5 mg/mL), dibasic sodium phosphate anhydrous (5 mg/mL), monobasic sodium phosphate monohydrate (2.5 mg/mL), polyethylene glycol 4000 (30 mg/mL), polysorbate 20 (12 mg/mL), sodium hydroxide to adjust pH, and water for injection. The drug product pH is 6.5 to 7.5.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
7PRINCIPAL DISPLAY PANEL - 39 mg/0.25 mL Syringe Kit
NDC 72526-105-11
Erzofri
39 mg/0.25 mL
For Single Use Only.
FOR INTRAMUSCULAR INJECTION ONLY.

8PRINCIPAL DISPLAY PANEL - 78 mg/0.5 mL Syringe Kit
NDC 72526-106-11
Erzofri
78 mg/0.5 mL
For Single Use Only.
FOR INTRAMUSCULAR INJECTION ONLY.

9PRINCIPAL DISPLAY PANEL - 117 mg/0.75 mL Syringe Kit
NDC 72526-107-11
Erzofri
117 mg/0.75 mL
For Single Use Only.
FOR INTRAMUSCULAR INJECTION ONLY.

10PRINCIPAL DISPLAY PANEL - 156 mg/mL Syringe Kit
NDC 72526-108-11
Erzofri
156 mg/mL
For Single Use Only.
FOR INTRAMUSCULAR INJECTION ONLY.

11PRINCIPAL DISPLAY PANEL - 234 mg/1.5 mL Syringe Kit
NDC 72526-109-11
Erzofri
234 mg/1.5 mL
For Single Use Only.
FOR INTRAMUSCULAR INJECTION ONLY.

12PRINCIPAL DISPLAY PANEL - 351 mg/2.25 mL Syringe Kit
NDC 72526-110-11
Erzofri
351 mg/2.25 mL
For Single Use Only.
FOR INTRAMUSCULAR INJECTION ONLY.
