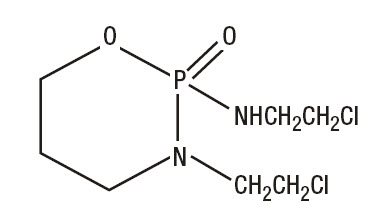
IFEX
What is IFEX (Ifosfamide)?
Approved To Treat
Related Clinical Trials
Summary: This single arm study is designed to demonstrate the feasibility of a radically different approach for an exceptionally high-risk subset of MES with widely metastatic disease (WMES). We incorporate the use of evolutionary principles that apply to species and population dynamics as related to adaptation and extinction to populations of cancer cells that similarly adapt and that we are attempting to...
Summary: The Glo-BNHL trial is trying to find better medicines for children and young people with B-cell non-Hodgkin Lymphoma (B-NHL) that does not go away (refractory B-NHL) or does but comes back again (relapsed B-NHL). B-NHL is a type of cancer that develops inside or outside of lymph nodes (glands) and organs such as the liver or spleen. Examples of B-NHL are Burkitt Lymphoma and Diffuse Large B Cell L...
Summary: This study utilizes a prospective, multicenter, randomized two-arm design to evaluate the efficacy and safety of the etoposide, cytarabine, and pegylated recombinant human granulocyte colony-stimulating factor (PEG-rhG-CSF) combination therapy (EAP regimen) in mobilizing hematopoietic stem cells in patients with non-Hodgkin's lymphoma (NHL). A total of 99 NHL patients will be enrolled as research ...
Related Latest Advances
Brand Information
- Myelosuppression can be severe and lead to fatal infections. Monitor blood counts prior to and at intervals after each treatment cycle
- Encephalopathy can be severe and may result in death. Monitor for CNS toxicity and discontinue treatment for encephalopathy
- Nephrotoxicity can be severe and result in renal failure. Hemorrhagic cystitis can be severe and can be reduced by the prophylactic use of mesna
- 1 gram single-dose vial for reconstitution
- 3 gram single-dose vial for reconstitution
- Known hypersensitivity to administration of ifosfamide.
- Urinary outflow obstruction.
- OSHA Hazardous Drugs. OSHA.
- IFEX (ifosfamide for injection)
- NDC 0338-3991-01 1-gram Single-Dose Vial
- NDC 0338-3993-01 3-gram Single-Dose Vial
- Advise patients that treatment with IFEX may cause myelosuppression which can be severe and lead to infections and fatal outcomes.
- Inform patients of the risks associated with the use of IFEX and plan for regular blood monitoring during therapy
- Inform patients to report fever or other symptoms of an infection
- Advise patients on the risks of bleeding and anemia
- Advise patients on the risk of encephalopathy and other neurotoxic effects with fatal outcome
- Inform patients that IFEX may impair the ability to operate an automobile or other heavy machinery
- Advise patients on the risk of bladder and kidney toxicity.
- Advise patients of the need to increase fluid intake and frequent voiding to prevent accumulation in the bladder
- Advise patients on the risk of cardiotoxicity and fatal outcome.
- Advise patients to report preexisting cardiac disease and manifestations of cardiotoxicity
- Advise patients on the risk of pulmonary toxicity leading to respiratory failure with fatal outcome.
- Inform patients to report signs and symptoms of pulmonary toxicity
- Advise patients on the risk of secondary malignancies due to therapy
- Advise patients on the risk of veno-occlusive liver disease
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during treatment with IFEX and for 12 months after the last dose
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with IFEX and for 6 months after the last dose
- Advise women not to breastfeed during treatment with IFEX and for 1 week after the last dose
- Advise females and males of reproductive potential that IFEX may cause temporary or permanent infertility
- Advise patients on the risk of alopecia, wound healing, and other serious skin and subcutaneous tissue disorders
- Advise patients that the therapy may cause gastrointestinal disorders and alcohol may increase nausea and vomiting
- Advise patients on the risk of stomatitis and the importance of proper oral hygiene
- Advise patients on the risk of eye disorders such as visual impairment, blurred vision, and eye irritation
- Advise patients on the risk of ear and labyrinth disorders such as deafness, vertigo, and tinnitus
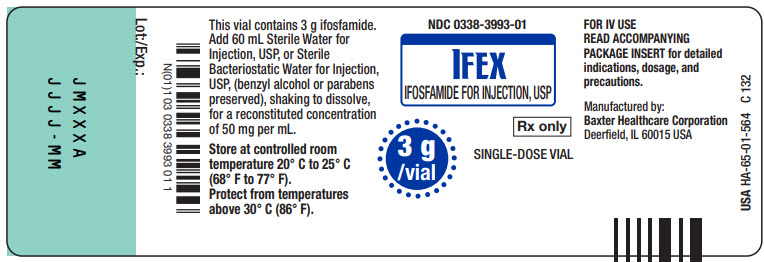
IFOSFAMIDE FOR INJECTION, USP
temperature 20° C to 25° C
(68° F to 77° F)
above 30° C (86° F).
PACKAGE INSERT for detailed
indications, dosage, and
precautions.


(ifosfamide for injection, USP)
FOR IV USE
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
INSERT for detailed indications,
dosage, and precautions.
temperature 20° C to 25° C
(68° F to 77° F). Protect from
temperatures above 30° C (86° F).
IFOSFAMIDE FOR INJECTION, USP
temperature 20° C to 25° C
(68° F to 77° F)
above 30° C (86° F).
READ ACCOMPANYING
PACKAGE INSERT for detailed
indications, dosage, and
precautions.
C 132


(ifosfamide for injection, USP)
FOR IV USE
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
INSERT for detailed indications,
dosage, and precautions.
temperature 20° C to 25° C
(68° F to 77° F). Protect from
temperatures above 30° C (86° F).
