AJOVY
What is AJOVY (Fremanezumab-Vfrm)?
Approved To Treat
Related Clinical Trials
Summary: The study aims to estimate treatment effects in a balanced placebo design (BPD) to specify to which extent contextual factors interact in preventive migraine treatment and influence adverse event occurrence in patients with chronic migraine. Using a clinical within-subjects design, patients with chronic migraine will receive four treatment conditions in a randomized order.
Summary: To evaluate the long-term safety of AJOVY in patients under actual use conditions and to specifically evaluate cardiovascular events. In addition, information on efficacy will be collected.
Summary: The main goal of this study is to determine whether there is a relationship between fremanezumab's ability to prevent migraine and improved sleep quality in migraine patients (fremanezumab is a FDA-approved humanized CGRP monoclonal antibody for the treatment of migraine). This is a within-person study design that examines treatment effects (changes) using high-resolution assessments. To complete ...
Related Latest Advances
Brand Information
- the preventive treatment of migraine in adults, and
- the preventive treatment of episodic migraine in pediatric patients who are 6 to 17 years of age and who weigh 45 kg or more.
- Injection: 225 mg/1.5 mL single-dose prefilled autoinjector
- Injection: 225 mg/1.5 mL single-dose prefilled syringe
- Hypersensitivity Reactions
- Hypertension
- Raynaud’s Phenomenon
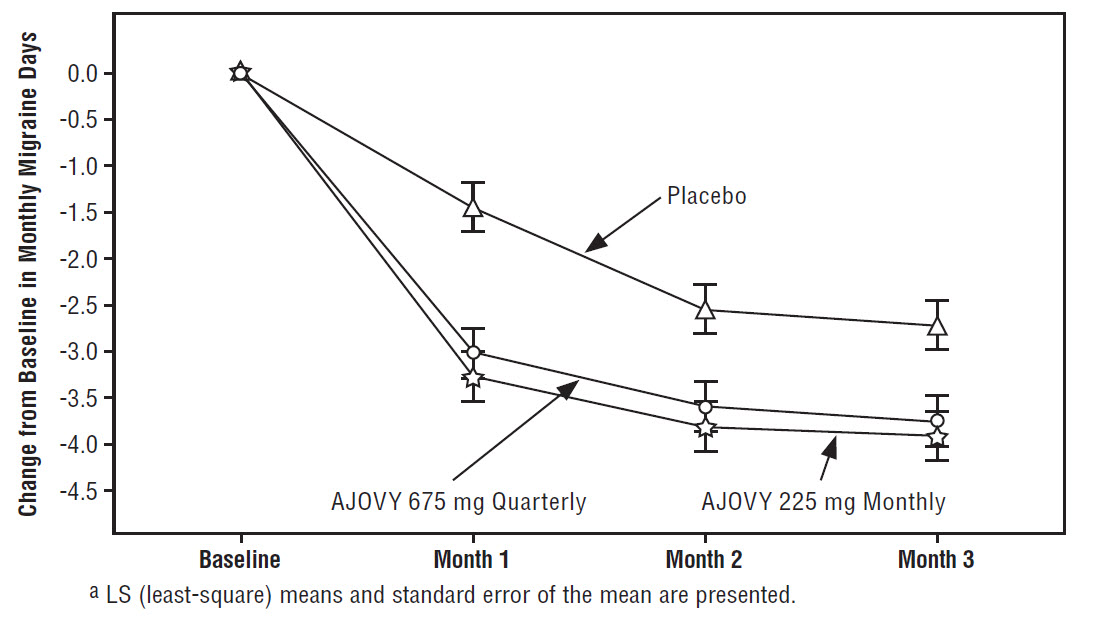
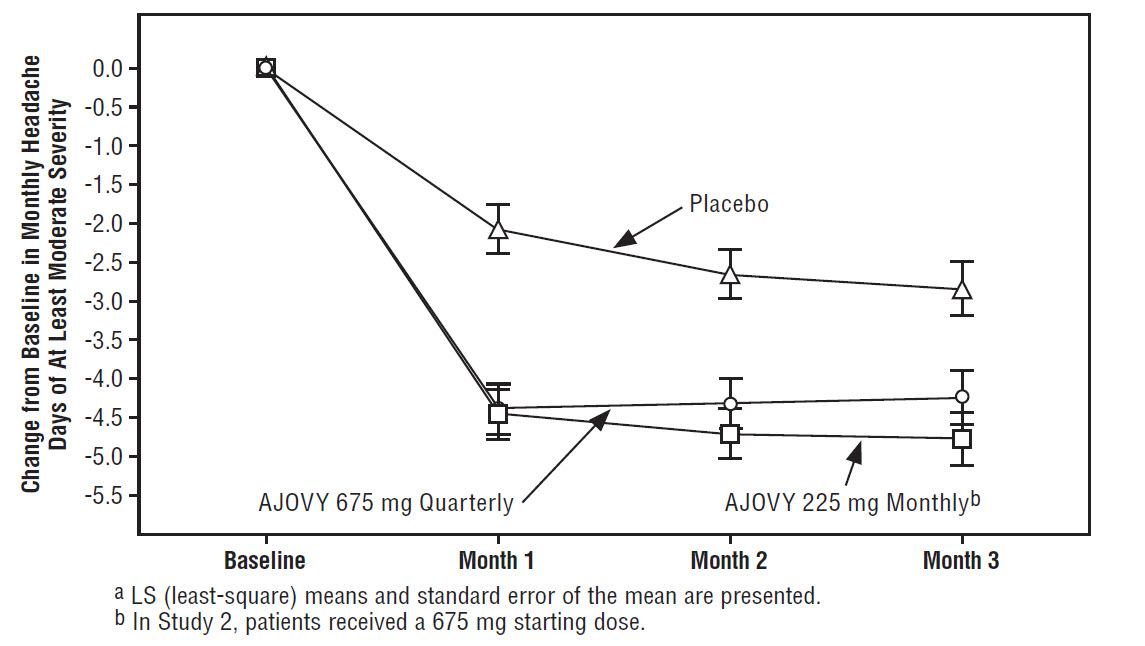
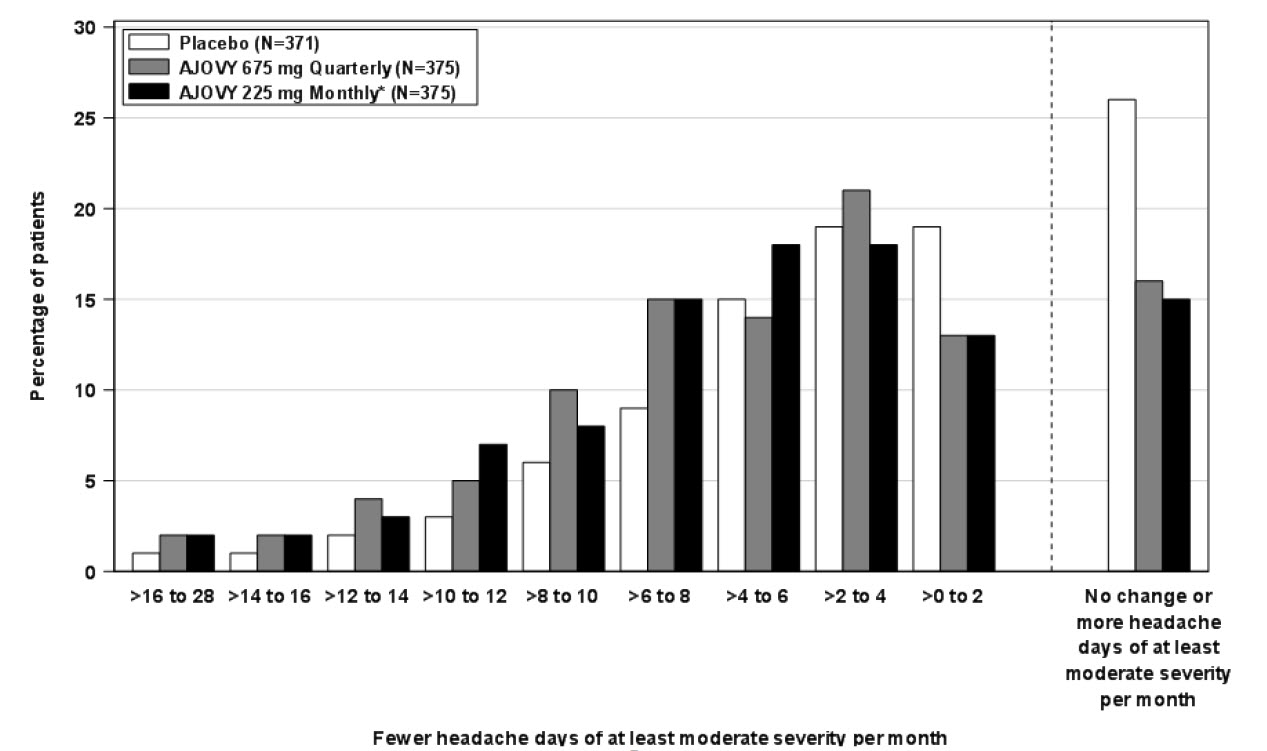
bUsed for chronic migraine diagnosis.
cUsed for primary endpoint analysis.
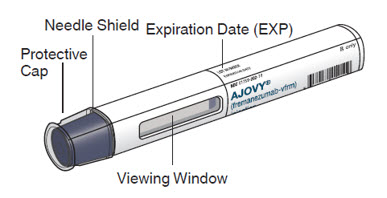
- AJOVY prefilled autoinjector is for single-time (one-time) use only. Put AJOVY in a FDA-cleared sharps disposal or puncture-resistant container right away after use. Do not throw away (dispose of) your used sharps disposal container in your household trash.
- Before injecting, let AJOVY sit at room temperature for 30 minutes.
- Keep AJOVY prefilled autoinjector out of the reach of small children.
- After you remove the protective cap from AJOVY, to prevent infection,
- Do not inject AJOVY in your veins (intravenously).
- Do not re-use your AJOVY prefilled autoinjector as this could cause injury or infection.
- Do not share your AJOVY prefilled autoinjector with another person. You may give another person an infection or get an infection from them.
- Store AJOVY in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep AJOVY in the carton it comes in to protect from light.
- If needed, AJOVY may be stored at room temperature up to 86°F (30°C) in the carton it comes in for up to 7 days. Do not use AJOVY if it has been out of the refrigerator for 7 days or longer. Throw away (dispose of) AJOVY in a sharps disposal or puncture-resistant container if it has been out of the refrigerator for 7 days or longer. Once stored at room temperature, do not place back in the refrigerator.
- Do not freeze. If AJOVY freezes, throw it away in a sharps disposal container.
- Keep AJOVY out of extreme heat and direct sunlight.
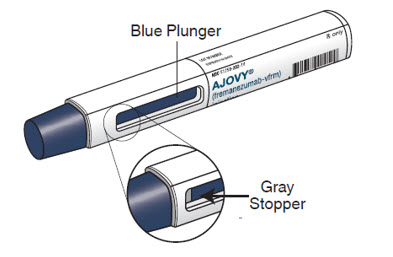

- The blue plunger moves down the viewing window during the injection. The blue plunger fills the window when the injection is complete.
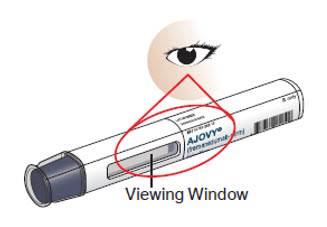
- When injecting AJOVY, hold the prefilled autoinjector so that your hand does not cover the viewing window.
- If your healthcare provider has prescribed 225 mg of AJOVY each month for you, give 1 injection each month, using a 225 mg prefilled AJOVY autoinjector.
- If your healthcare provider has prescribed 675 mg of AJOVY every 3 months for you, give 3 separate injections, one after another, using a different 225 mg prefilled AJOVY autoinjector for each injection. Give these injections 1 time every 3 months.
- You may need to use more than 1 prefilled autoinjector depending on your prescribed dose.
- Remove the autoinjector from the carton (see Figure C).
- Do not shake the prefilled autoinjector at any time, as this could affect the way the medicine works.
- Gather the following supplies (see Figure D) and the number of AJOVY 225 mg prefilled autoinjectors you will need to give your prescribed dose:
- Place the supplies you have gathered on a clean, flat surface.
- Wait for 30 minutes to allow the medicine to reach room temperature.
- Do not leave the prefilled autoinjector in direct sunlight.
- Do not warm up the AJOVY prefilled autoinjector using a heat source such as hot water or a microwave.
- Wash your hands with soap and water and dry well with a clean towel. Be careful not to touch your face or hair after washing your hands.
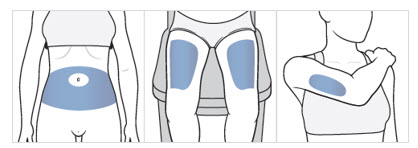
- Choose an injection area from the following areas (see Figure F):
- your
- the
- the
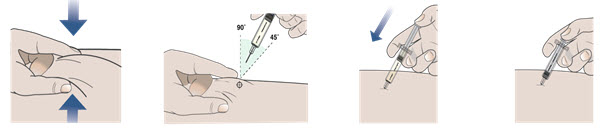
- Clean the chosen injection area using a new alcohol swab. Let your skin dry.
- Do not inject AJOVY into an area that is tender, red, bruised, callused, tattooed, hard, or that has scars or stretch marks.
- Do not inject AJOVY in the same injection site that you inject other medicine.
- If you want to use the same injection area for the 3 separate injections needed for the 675 mg dose, make sure the second and third injections are not at the same spot you used for the other injections.
- Pick up the prefilled autoinjector in 1 hand.
- Hold the prefilled autoinjector as shown in Figure G and pull the protective cap straight off with your other hand. Do not twist.
- Throw away the protective cap right away.
- Do not put the protective cap back on the prefilled autoinjector, to avoid injury and infection.
- 10.1 Place the prefilled autoinjector at a 90 degree angle against your skin at the injection site you have cleaned (see Figure H).
- Use a clean, dry cotton ball, or gauze pad to
- Do not rub the injection site.
- Do not re-use the prefilled autoinjector.
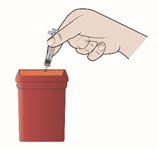
- Put your used prefilled autoinjectors in a FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) prefilled autoinjectors in your household trash. Do not recycle your used sharps disposal container.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used autoinjectors. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- AJOVY prefilled syringe is for single-time (one-time) use only. Put AJOVY in a FDA-cleared sharps disposal or puncture-resistant container right away after use. Do not throw away (dispose of) your used sharps disposal container in your household trash.
- Before injecting, let AJOVY sit at room temperature for 30 minutes.
- Keep AJOVY prefilled syringe out of the reach of small children.
- After you remove the needle cap from AJOVY, to prevent infection,
- Do not pull back on the plunger at any time, as this can break the prefilled syringe.
- Do not inject AJOVY in your veins (intravenously).
- Do not re-use your AJOVY prefilled syringe, as this could cause injury or infection.
- Do not share your AJOVY prefilled syringe with another person. You may give another person an infection or get an infection from them.
- Store AJOVY in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep AJOVY in the carton it comes in to protect from light.
- If needed, AJOVY may be stored at room temperature up to 86°F (30°C) in the carton it comes in for up to 7 days. Do not use AJOVY if it has been out of the refrigerator for 7 days or longer. Throw away (dispose of) AJOVY in a sharps disposal or puncture-resistant container if it has been out of the refrigerator for 7 days or longer. Once stored at room temperature, do not place back in the refrigerator.
- Do not freeze. If AJOVY freezes, throw it away in a sharps disposal container.
- Keep AJOVY out of extreme heat and direct sunlight.
- Do not shake AJOVY.


- If your healthcare provider has prescribed 225 mg of AJOVY each month for you, give 1 injection each month using a 225 mg prefilled AJOVY syringe.
- If your healthcare provider has prescribed 675 mg of AJOVY every 3 months for you, give 3 separate injections, one after another, using a different 225 mg prefilled AJOVY syringe for each injection. Give these injections 1 time every 3 months.
- You may need to use more than 1 prefilled syringe depending on your prescribed dose.
- Hold the prefilled syringe (see Figure C).
- Remove the syringe from the carton.
- Do not shake the prefilled syringe at any time, as this could affect the way the medicine works.
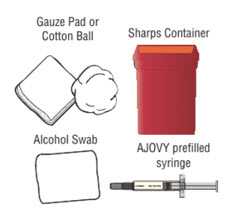
- Gather the following supplies (see Figure D) and the number of AJOVY 225 mg prefilled syringes you will need to give your prescribed dose:

- Place the supplies you have gathered on a clean, flat surface.
- Wait for 30 minutes to allow the medicine to reach room temperature.
- Do not leave the prefilled syringe in direct sunlight.
- Do not warm up the AJOVY prefilled syringe using a heat source such as hot water or a microwave.
- Wash your hands with soap and water and dry well with a clean towel. Be careful not to touch your face or hair after washing your hands.
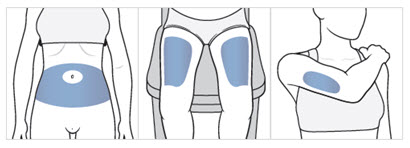
- Choose an injection area from the following areas (see Figure F):
- your
- the
- the

- Clean the chosen injection area using a new alcohol swab. Let your skin dry.
- Do not inject AJOVY into an area that is tender, red, bruised, callused, tattooed, hard, or that has scars or stretch marks.
- Do not inject AJOVY in the same injection site that you inject other medicine.
- If you want to use the same injection area for the 3 separate injections needed for the 675 mg dose, make sure the second and third injections are not at the same spot you used for the other injections.
- Pick up the body of the prefilled syringe with 1 hand.
- Pull the needle cap straight off with your other hand (see Figure G). Do not twist.
- Throw away the needle cap right away.
- Do not put the needle cap back on the prefilled syringe, to avoid injury and infection.
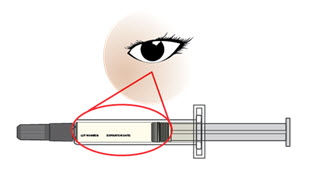
- After you have injected all of the medicine,
- Do not recap the needle at any time to avoid injury and infection.
- Use a clean, dry cotton ball or gauze to
- Do not rub the injection site.
- Do not re-use the prefilled syringe.

- Put your used prefilled syringes, needles, and sharps in a FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) loose needles, syringes, or prefilled syringes in your household trash. Do not recycle your used sharps disposal container.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Rx onlyAJOVYFOR SUBCUTANEOUS USE ONLYOne single-dose prefilled syringe
Store in refrigerator at 36°F to 46°F (2° to 8°C) in the original carton to protect from light. DO NOT FREEZE. DO NOT SHAKE. If needed, AJOVY® may be kept at room temperature up to 86°F (30°C) for 7 days. Once stored at room temperature, do not place back in the refrigerator. Discard after 7 days.

Rx only
One single-dose prefilled autoinjector
Store in refrigerator at 36°F to 46°F (2° to 8°C) in the original carton to protect from light. DO NOT FREEZE. DO NOT SHAKE. If needed, AJOVY® may be kept at room temperature up to 86°F (30°C) for 7 days. Once stored at room temperature, do not place back in the refrigerator. Discard after 7 days.

Rx only
3 x 225 mg/1.5 mL single-dose prefilled autoinjectors
Store in refrigerator at 36°F to 46°F (2° to 8°C) in the original carton to protect from light. DO NOT FREEZE. DO NOT SHAKE. If needed, AJOVY® may be kept at room temperature up to 86°F (30°C) for 7 days. Once stored at room temperature, do not place back in the refrigerator. Discard after 7 days.
