Siliq
What is Siliq (Brodalumab)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to test the safety and effectiveness of using brodalumab in patients who develop side effects from cancer immune therapy. Immune-related side effects are due to activation of the immune system in patients who previously received immunotherapy and the goal of this study is to help better control these side effects. Brodalumab is often used to treat patients with autoimm...
Summary: Biologics, such as brodalumab, are currently the most effective treatment option for patients with moderate-to-severe psoriasis. But they are costly for health care systems and prescribed according to a 'one dose fits all' dosing regimen, leading to potential over- and undertreatment. Within this study we aim to investigate the predictive value of early serum trough levels of brodalumab and determ...
Related Latest Advances
Brand Information
- Crohn’s disease because SILIQ may cause worsening of disease [see Warnings and Precautions (
- Clinically significant hypersensitivity to brodalumab or to any of the excipients in SILIQ or component of the container. Hypersensitivity reactions, including anaphylaxis, have been reported with postmarket use of SILIQ [see Warnings and Precautions (
- Suicidal Ideation and Behavior
- Hypersensitivity Reactions
- Infections [
- Crohn’s Disease
- NDC 0187-0004-02: Carton of two 210 mg/1.5 mL single-dose prefilled syringes
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light and physical damage during storage.
- When necessary, prefilled syringes can be stored at room temperature up to a maximum of 77°F (25°C) in the original carton for a maximum single period of 14 days with protection from light and sources of heat. Once the prefilled syringe has reached room temperature, do not place back into the refrigerator. Discard after 14 days at room temperature.
- Do not freeze.
- Do not shake.
- Patients must enroll in the program
- Patients will be given a SILIQ Patient Wallet Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation. Advise the patient to show the SILIQ Patient Wallet Card to other treating healthcare providers.
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bausch Health Ireland, Limited
Dublin, Leinster, Ireland 24
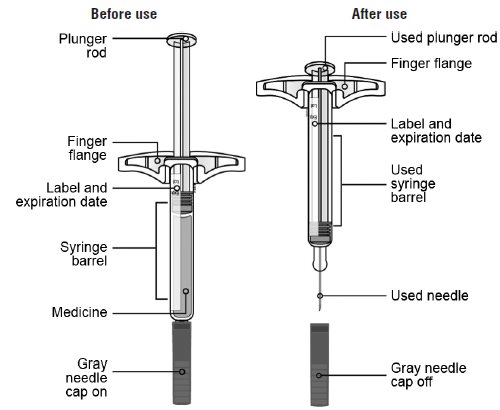
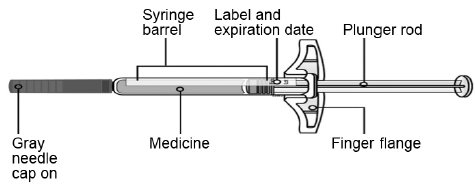
- Store SILIQ prefilled syringe in the refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light and physical damage.
- If needed, SILIQ prefilled syringe may be stored at room temperature up to 77°F (25°C) for up to 14 days.
- Throw away SILIQ prefilled syringe that has been stored at room temperature after 14 days.
- Protect SILIQ prefilled syringe from heat.
- Do notfreeze.
- Do notshake.
- Keep SILIQ prefilled syringe in the original carton to protect from light and physical damage.
- Keep SILIQ prefilled syringe and all medicines out of the reach of children.
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- Do notuse a SILIQ prefilled syringe after the expiration date on the label.
- Do notshake the SILIQ prefilled syringe.
- Do notremove the gray needle cap from the SILIQ prefilled syringe until you are ready to inject.
- Do notuse a SILIQ prefilled syringe if it has been dropped on a hard surface. Part of the SILIQ prefilled syringe may be broken even if you cannot see the break. Use a new SILIQ prefilled syringe, and call 1-800-321-4576.
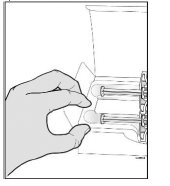
- Do notgrab the plunger rod.
- Do notgrab the gray needle cap.
- Do notremove the gray needle cap until you are ready to inject.
- Do notput the prefilled syringe back in the refrigerator after it has reached room temperature.
- Do nottry to warm the prefilled syringe by using a heat source such as hot water or microwave.
- Do notleave the prefilled syringe in direct sunlight.
- Do notshake the prefilled syringe.
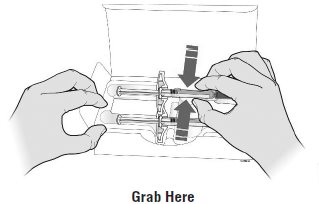
- Do notuse the syringe if:
- The medicine is cloudy or discolored or contains flakes or particles.
- Any part appears cracked or broken.
- The gray needle cap is missing or not securely attached.
- The expiration date printed on the label has passed.
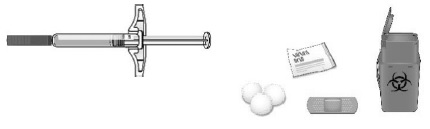
- Prefilled syringe
- Alcohol wipes
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container
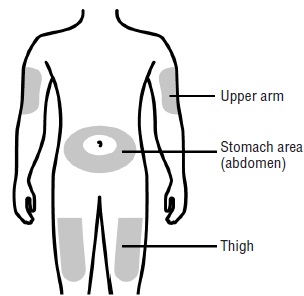
- Your thigh
- Stomach area (abdomen), except for a
- Outer area of upper arm (only if someone else is giving you the injection)
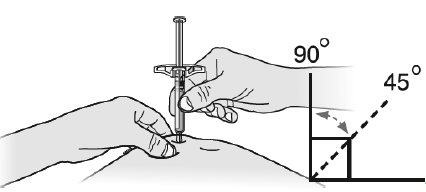
- Do nottouch this area again before injecting.
- Choose a different site each time you give yourself an injection. If you want to use the same injection site, make sure it is not the same spot on the injection site that you used for a previous injection.
- Do notinject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
- Avoid injecting directly into raised, thick, red, or scaly skin patch or lesion.
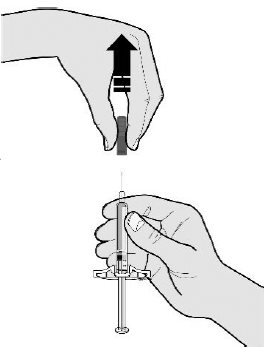
- Do nottwist or bend the gray needle cap.
- Do notput the gray needle cap back onto the prefilled syringe.
- Do notremove the gray needle cap from the prefilled syringe until you are ready to inject.
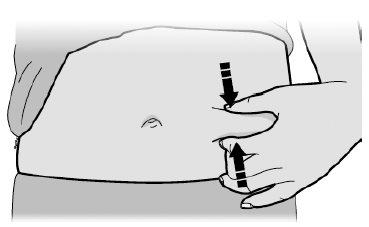
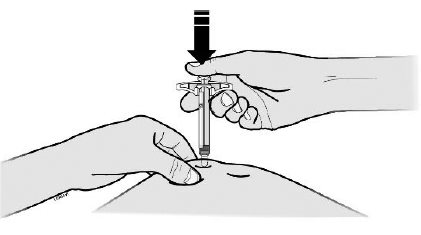
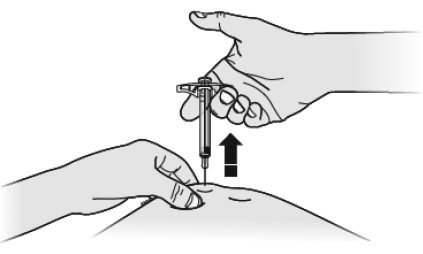
- Put the used SILIQ syringe in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do notreuse the syringe.
- Do notrecycle the syringe or sharps disposal container or throw them into household trash.

Contains 2 Single-Dose Prefilled Syringes
NDC0187-0004-02
SILIQ ®
(brodalumab)
Injection
210 mg/1.5 mL
Sterile Solution – No Preservative
KEEP OUT OF REACH OF CHILDREN.



