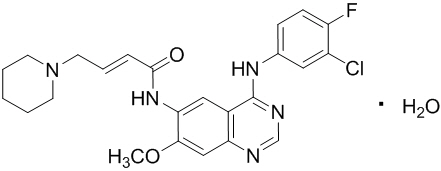
Vizimpro
What is Vizimpro (Dacomitinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Vizimpro will be approved for the treatment of EGFR NSLCL 1L in Korea. In accordance with the Standards for Re-examination of New Drug, it is required to conduct a PMS. Post marketing surveillance is required to determine any problems or questions associated with Vizimpro after marketing in Korea, with regard to the following clauses under conditions of general clinical practice. Therefore, throug...
Summary: A Phase II, open label, non-randomized, multiple-arm, single-center clinical trial in patients with advanced rare solid tumors who failed to standard treatment.
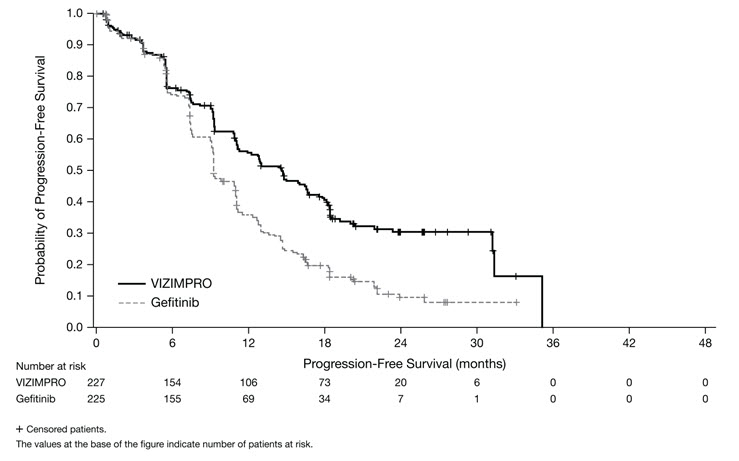
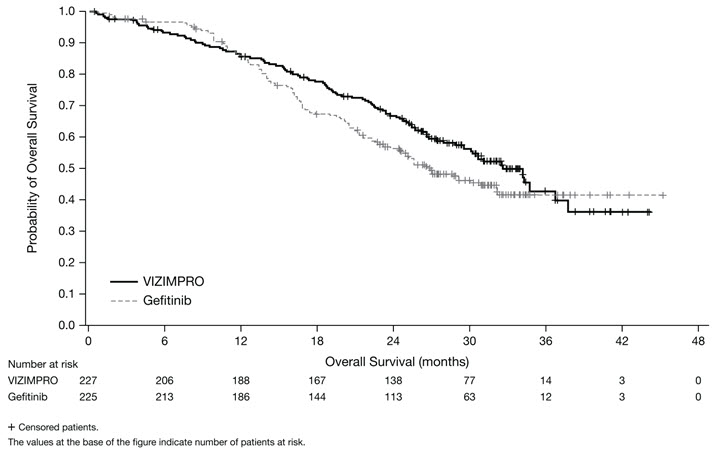
Summary: The purpose of this study is to evaluate the optimal sequence of EGFR-inhibitors in lung cancer patients with EGFR-positive tumors not amenable for curative treatment. Life quality, adverse effects and tumor response will be evaluated and analyses of obtained blood and tumor samples will be performed to identify molecular profiles and biomarkers that can be used for treatment decisions.
Related Latest Advances
Brand Information
- 45 mg: blue film-coated, immediate release, round biconvex tablet, debossed with "Pfizer" on one side and "DCB45" on the other side.
- 30 mg: blue film-coated, immediate release, round biconvex tablet, debossed with "Pfizer" on one side and "DCB30" on the other side.
- 15 mg: blue film-coated, immediate release, round biconvex tablet, debossed with "Pfizer" on one side and "DCB15" on the other side.
- Interstitial Lung Disease
- Diarrhea
- Dermatologic Adverse Reactions