Generic Name
Tc-99M Exametazime
Brand Names
Ceretec, Drax Exametazime
FDA approval date: December 30, 1988
Classification: Radioactive Diagnostic Agent
Form: Injection, Kit
What is Ceretec (Tc-99M Exametazime)?
Ceretec is a radioactive diagnostic agent, indicated in adults and pediatric patients age 2 to 17 for: Leukocyte Labeled Scintigraphy – As an adjunct in the localization of intraabdominal infection and inflammatory bowel disease.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
CERETEC (TECHNETIUM TC-99M EXAMETAZIME)
1DESCRIPTION
The Ceretec kit is supplied as a pack of 5 vials for use in the preparation of a technetium Tc99m exametazime intravenous injection as a diagnostic radiopharmaceutical for use as an adjunct in the detection of altered regional cerebral perfusion and for the radiolabeling of autologous leukocytes. Each vial of Ceretec contains a pre-dispensed sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime [(RR,SS)-4.8-diaza-3,6,6,9-tetramethylundecane-2, 10-dione bisoxime], 7.6 µg stannous chloride dihydrate (minimum stannous tin 0.6 µg; maximum total stannous and stannic tin 4.0 µg per vial) and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative.
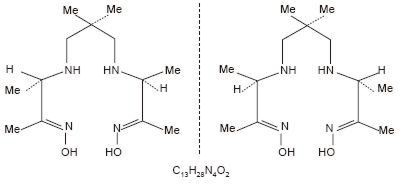
Prior to publication of the USAN, exametazime was formerly known as hexamethylpropylene amine oxime (HM-PAO). The name HM-PAO appears in many publications.
The structural formula of exametazime is:
When sterile pyrogen-free sodium pertechnetate Tc99m in isotonic saline is added to the vial of Ceretec, a Tc99m complex of exametazime is formed.
Administration is by intravenous injection for diagnostic use.
1.1Physical Characteristics
Technetium Tc99m decays by isomeric transition with a physical half-life of 6.03 hours.
1.2External Radiation
The specific gamma ray constant for technetium Tc99m is 206 microCoulomb kg-1/37 MBq-h, (0.8 R/millicurie-h) at 1 cm. The first half-value thickness of lead (Pb) for technetium Tc99m is 0.2 mm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of a 2.7 mm thickness of Pb will decrease the external radiation exposure by a factor of 1,000.
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals relative to the time of calibration are shown in Table 3.
2CLINICAL TRIALS
Two clinical trials were performed in a total of 88 patients who had suspected intra-abdominal infection or inflammation. Subjects received both Tc99m labeled leukocytes and In-111 labeled leukocytes. Images were obtained at 2 and 30 minutes and at 2 and 4 hours and 24 hours. In two other clinical trials, in a total of 127 patients with suspected abdominal inflammation or infection received Tc99m labeled leukocytes. Imaging was at 24 hours in one study and at 1, 3 and 24 hours in the other. In all 4 studies, images were blindly evaluated and the findings were confirmed by surgery, biopsy or other clinical data.
Based on the above 4 studies, between 2 to 4 hours Tc99m labeled leukocytes had 95-100% sensitivity and 62-85% specificity with similar numbers of false positive and false negative findings. The value of the 24 hour Tc99m labeled leukocyte images is inconsistent. In all studies the false positive and false negatives relate to the bowel background, the location of the site of infection/inflammation and whether or not it is contiguous with the bowel. The 24 hour films should be interpreted with great caution because of a high bowel background; false negatives were noted in both Tc99m and In-111 labeled leukocytes.
Other studies suggest that the interpretation of the images could be affected by the presence of tumors, infarction and peritonitis, etc. Liver abscess may be missed because of the bowel background. Caution should be exercised in making the final diagnosis.
3INDICATIONS AND USAGE
Technetium Tc99m exametazime scintigraphy may be useful as an adjunct in the detection of altered regional cerebral perfusion in stroke.
Tc99m exametazime is indicated for leukocyte labeled scintigraphy as an adjunct in the localization of intra-abdominal infection and inflammatory bowel disease.
4CONTRAINDICATIONS
None known.
5PRECAUTIONS
As with any injected product, acute hypersensitivity or allergic reactions are possible. Limited reports have been received of hypersensitivity reactions following administration of Tc99m labeled leukocytes prepared using Tc99m exametazime. However, the materials used in leukocyte cell separation may cause hypersensitivity reactions. It is essential that cells are washed free of sedimentation agents before they are reinjected into the patient.
In case of side effects following administration of radiopharmaceuticals, users should ensure the availability of appropriate medical treatment at the time of administration of any radiopharmaceutical to the patient.
A thorough knowledge of the normal distribution of intravenously administered technetium Tc99m exametazime injection is essential in order to interpret pathologic studies accurately. Caution should be exercised in making the final diagnosis. Results can be affected by the presence of tumor, infarction, peritonitis, non-gastrointestinal or bony sites of inflammatory cell collections.
The contents of the Ceretec vial are not radioactive. After the sodium pertechnetate Tc99m is added, the product is radioactive and adequate shielding of the final preparation must be maintained. The contents of the Ceretec vial are intended only for use in preparation of technetium Tc99m exametazime injection and are NOT to be administered directly to the patient.
5.1General
The contents of the Ceretec vial are sterile and pyrogen free. The vial contains no bacteriostatic preservative. It is essential that the user follow the directions carefully and adhere to strict aseptic procedures during preparation of the radiopharmaceutical.
Radiopharmaceuticals should be used only by or under the control of physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
To minimize radiation dose to the bladder, the patient should be encouraged to void when the examination is completed and as often thereafter as possible. Adequate hydration should be encouraged to permit frequent voiding.
5.2Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies have not been performed to evaluate carcinogenic potential or whether exametazime affects fertility in males or females. When evaluated in the Ames test, exametazime increased the apparent rate of gene mutation in the TA100 strain of S. typhimurium. Exametazime did not cause chromosomal aberrations
5.3Pregnancy Category C
Animal reproduction studies have not been conducted with Tc99m exametazime. It is also not known whether Tc99m exametazime can cause fetal harm when administered to a pregnant woman or if it can affect reproductive capacity. Therefore, Tc99m exametazime should not be administered to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
5.4Nursing Mothers
Technetium Tc99m is excreted in human milk during lactation. It is not known whether exametazime is excreted in human milk. Therefore, formula feedings should be substituted for breast feeding for 60 hours.
5.5Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
5.6Geriatric Use
Clinical studies of Ceretec™ did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
6ADVERSE REACTIONS
Rash with generalized erythema, facial edema and fever has been reported in less than 1% of patients. A transient increase in blood pressure was seen in 8% of patients.
7DOSAGE AND ADMINISTRATION
Tc99m labeled leukocytes for adjunctive localization of intra-abdominal infection or inflammation.
The normal adult (70 kg) dose is 0.259-0.925 GBq (7-25 mCi) as Tc99m labeled leukocytes by intravenous injection. Optimal planar imaging is between 2-4 hours.
7.1Cerebral Scintigraphy
The recommended dose range for i.v. administration, of reconstituted sodium pertechnetate Tc99m exametazime in the average adult (70 kg) is 370-740 MBq (10-20 mCi).
Dynamic imaging may be performed between 0 to 10 minutes following injection. Static imaging may be performed from 15 minutes up to 6 hours after injection.
8RADIATION DOSIMETRY
Based on human data, the absorbed radiation doses to an average human adult (70 kg) from an intravenous injection of this product are estimated below. The values are listed as µGy/MBq, rads/mCi with urination every 2 hours. Bladder wall dose is 19 µGy/MBq, 0.07 rads/mCi with 4 hour urination and 89 µGy/MBq, 0.33 rads/mCi with no urination.
9HOW SUPPLIED
The kit comprises 5 individual vials of sterile, non-pyrogenic, freeze-dried mixture of exametazime, stannous chloride dihydrate and sodium chloride, 5 radiation labels, 5 radiochemical purity worksheets, 5 labeling efficiency worksheets, 1 leukocyte labeling schematic and 1 package insert.
NDC 17156-022-05
9.1Storage
Store the kit at 15°-25°C (59°-77°F).
Store the formulated drug for up to 30 minutes at 20°-25°C (68°-77°F) using appropriate radiation shielding.
This reagent kit is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant to 32 Ill. Code Adm. Section, Section 330.260(a) and 335.4010 or under equivalent licenses of the U.S. Nuclear Regulatory Commission, or an Agreement State.
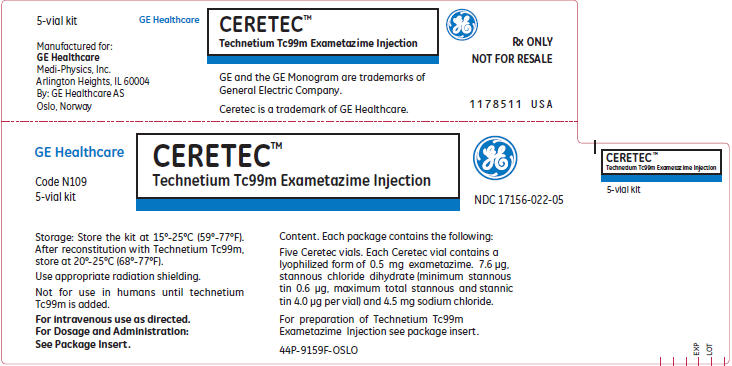
10PRINCIPAL DISPLAY PANEL - 5 Vial Kit
GE Healthcare
CERETEC™
Code N109
NDC 17156-022-05
Storage: Store the kit at 15°-25°C (59°-77°F).
Use appropriate radiation shielding.
Not for use in humans until technetium
For intravenous use as directed.
Content. Each package contains the following:
Five Ceretec vials. Each Ceretec vial contains a
For preparation of Technetium Tc99m
44P-9159F-OSLO