SymlinPen
What is SymlinPen (Pramlintide Acetate)?
For people living with type 1 or type 2 diabetes, managing blood sugar levels with insulin is a daily reality. Yet, even with diligent monitoring and insulin use, achieving stable glucose control can be a constant challenge, especially after meals. Those frustrating post-meal spikes can feel unpredictable and difficult to manage. This is where a unique, adjunctive therapy called SymlinPen (pramlintide acetate) can play a supportive role.
SymlinPen is not insulin, nor is it a replacement for it. Instead, it is an injectable prescription medication that works alongside insulin to improve glucose control. It belongs to a class of drugs known as amylin analogs, meaning it is a synthetic version of a natural hormone called amylin. In people without diabetes, amylin and insulin are released together by the pancreas to manage blood sugar. SymlinPen is designed for patients who use mealtime insulin but still struggle to reach their blood sugar targets, offering another tool to help smooth out the peaks and valleys of daily glucose management.
What does SymlinPen do?
SymlinPen is approved by the U.S. Food and Drug Administration (FDA) as an add-on treatment for adults with either type 1 or type 2 diabetes who use mealtime insulin. Its primary purpose is to improve glycemic control, particularly after eating.
By adding SymlinPen to their insulin regimen, patients may experience:
- Reduced Post-Meal Blood Sugar Spikes: It helps to prevent the sharp rise in glucose that can occur after eating a meal.
- Lower Hemoglobin A1c Levels: By improving overall glucose control, it can help lower A1c, which is a key measure of long-term blood sugar management.
- Potential for Reduced Mealtime Insulin Doses: Because it helps manage post-meal glucose, some patients may find they need less mealtime insulin.
- Modest Weight Loss: It can help promote a feeling of fullness, which may lead to eating less and a slight reduction in body weight.
Clinical studies have shown that when added to insulin therapy, SymlinPen significantly improves A1c levels and reduces post-meal glucose excursions compared to insulin alone, providing a valuable option for fine-tuning diabetes care (AstraZeneca Pharmaceuticals LP, 2021).
How does SymlinPen work?
SymlinPen works by mimicking the actions of the natural hormone amylin. In a person without diabetes, the pancreas releases both insulin and amylin in response to a meal. While insulin helps cells absorb sugar from the blood, amylin helps to regulate the speed at which sugar enters the blood in the first place. Many people with diabetes either don’t produce amylin or don’t produce enough of it.
SymlinPen helps manage blood sugar by:
- Slowing gastric emptying: Delays carbohydrate absorption, preventing rapid blood sugar spikes after meals.
- Suppressing glucagon secretion: Blocks the release of stored sugar from the liver, reducing excess blood sugar.
- Promoting satiety: Increases feelings of fullness, aiding in smaller meal portions and calorie reduction.
By performing these three actions, SymlinPen provides a multi-faceted approach to improving post-meal blood sugar control that complements the action of insulin.
SymlinPen side effects
The most significant risk associated with SymlinPen is a boxed warning, the FDA’s strongest warning, for the risk of severe hypoglycemia (dangerously low blood sugar). This risk is highest when SymlinPen is used with insulin.
Severe hypoglycemia risk is highest within three hours post-injection. When starting SymlinPen, reduce your usual mealtime insulin dose by 50% as instructed by your doctor. Frequent blood sugar monitoring, especially pre- and post-meals, is crucial to prevent low blood sugar.
The most common side effect of SymlinPen is nausea, especially when starting the medication. This is often mild to moderate and tends to decrease over time. Other common side effects may include:
- Decreased appetite
- Vomiting
- Headache
- Injection site reactions (redness, swelling)
Do not use SymlinPen if you have gastroparesis or hypoglycemia unawareness. Regular blood sugar testing is essential. Seek immediate medical attention for severe, unmanageable hypoglycemia symptoms like confusion, seizures, or loss of consciousness.
SymlinPen dosage
SymlinPen is an injectable medication that comes in a prefilled pen device. It is administered as a subcutaneous injection (an injection under the skin) into the abdomen or thigh.
Crucially, SymlinPen is injected immediately before major meals (meals containing at least 250 calories or 30 grams of carbohydrates). It is a separate injection from insulin and should never be mixed in the same syringe.
Your doctor will gradually increase your SymlinPen dose to minimize nausea. Close monitoring of blood glucose is essential, especially when starting or changing doses of SymlinPen or insulin. Mealtime insulin doses must be reduced at the start of SymlinPen, and your doctor will guide further adjustments.
Does SymlinPen have a generic version?
No, there is currently no generic version of SymlinPen (pramlintide acetate) available. However, international versions may exist in other markets. SymlinPen is a biologic medication, and a biosimilar (the equivalent of a generic) has not been approved by the FDA. Therefore, it is only available under the brand name SymlinPen.
Conclusion
For individuals with type 1 or type 2 diabetes who are already using mealtime insulin but still struggle with post-meal blood sugar control, SymlinPen offers a unique and effective adjunctive therapy. By mimicking the natural hormone amylin, it helps to smooth out glucose spikes, lower A1c, and may assist with weight management.
However, its use requires a significant commitment to safety. The risk of severe hypoglycemia is real and must be managed through careful insulin dose adjustments and frequent blood sugar monitoring. When used under the close guidance of a healthcare provider, SymlinPen can be a valuable addition to a comprehensive diabetes management plan, helping you achieve better control and take another positive step on your health journey.
References
- AstraZeneca Pharmaceuticals LP. (2021). SYMLINPEN® (pramlintide acetate) injection Prescribing Information. U.S. Food and Drug Administration. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021342s031lbl.pdf
- Mayo Clinic. (2024). Pramlintide (Subcutaneous Route). Retrieved from https://www.mayoclinic.org/drugs-supplements/pramlintide-subcutaneous-route/side-effects/drg-20065611
Approved To Treat
Top Global Experts
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
- 1.5 mL disposable multidose SymlinPen
- 2.7 mL disposable multidose SymlinPen
- serious hypersensitivity reaction to SYMLIN or to any of its product components.
- hypoglycemia unawareness.
- confirmed gastroparesis.
- Injection site reactions
- Pancreatitis
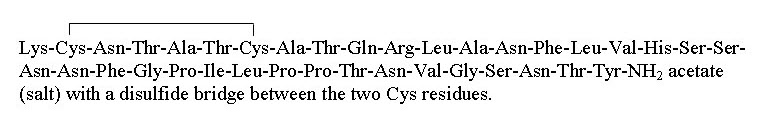
- SymlinPen
- SymlinPen
(pramlintide acetate)
- severe low blood sugar (hypoglycemia). Even when SYMLIN is carefully added to your mealtime insulin therapy, your blood sugar may drop too low, especially if you have type 1 diabetes. If this severe low blood sugar (hypoglycemia) happens, it is seen within 3 hours after a SYMLIN injection. Symptoms of severe low blood sugar and low blood sugar include:
- lightheadedness
- dizziness
- shakiness
- sweating
- hunger
- fast heartbeat
- trouble concentrating or confusion
- change in vision
- headache
- irritability
- drowsiness
- You have a higher chance of getting severe low blood sugar if you:
- do not follow your healthcare provider’s instructions to reduce your insulin use before meals
- use more SYMLIN or insulin than prescribed by your healthcare provider
- change your insulin dose without checking your blood sugar
- eat less food than your usual meal
- are sick and cannot eat
- are more active than usual
- have a low blood sugar level before eating
- drink alcohol
- SYMLIN is used with insulin to lower blood sugar, especially high blood sugar that happens after meals.
- SYMLIN is taken at mealtimes. The use of SYMLIN does not replace your daily insulin but may lower the amount of insulin you need, especially before meals.
- are allergic to SYMLIN or any ingredients in SYMLIN. See the end of this Medication Guide for a complete list of ingredients in SYMLIN.
- cannot tell when your blood sugar is low (hypoglycemia unawareness)
- have a stomach problem called gastroparesis. This is when your stomach does not empty as fast as it should.
- are pregnant or plan to become pregnant. It is not known if SYMLIN will harm your unborn baby. You and your healthcare provider should decide how to best control your blood sugar levels during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if SYMLIN passes into your breast milk. You and your healthcare provider should decide about the best way to feed your baby if you are using SYMLIN.
- Read the “Instructions for Use” and the Medication Guide and that come with your SYMLIN for information about the right way to use SYMLIN.
- Use SYMLIN exactly as your healthcare provider tells you to use it.
- Your healthcare provider will tell you how much SYMLIN to use and when to use it.
- Your healthcare provider may change your dose if needed.
- If you stop taking SYMLIN for any reason, such as surgery or illness, talk to your healthcare provider about how to re-start SYMLIN.
- To reduce the risk of hypoglycemia, it is important that you plan your meals and physical activity every day while you use SYMLIN. Plan for what you will eat and when you will eat your meals.
- The amount of SYMLIN you use will depend on whether you have type 1 or type 2 diabetes.
- The way you inject SYMLIN is similar to the way you inject insulin. Inject SYMLIN under the skin of your stomach area (abdomen) or upper leg (thigh). Inject SYMLIN at a site that is more than 2 inches away from your insulin injection. Do not inject SYMLIN and insulin in the same site.
- To help reduce the chances of getting a reaction at the injection site, allow SYMLIN to come to room temperature before injecting.
- Use a new needle for each SYMLIN injection.
- Never mix SYMLIN and insulin. Insulin can affect SYMLIN when they are mixed together.
- Do not use SYMLIN if the liquid looks cloudy.
- If you take more than your prescribed dose of SYMLIN, you may get nauseous or vomit, and may not be able to eat the amount of food you usually eat. If you take more SYMLIN than your prescribed dose, pay careful attention to the amount of insulin you use because you may be at more risk for low blood sugar. Contact your healthcare provider for guidance.
- If you miss or forget a dose of SYMLIN, wait until the next meal and take your usual dose of SYMLIN at that meal.
- Do not share your SymlinPen with other people, even if the needle is changed.You may give other people a serious infection or get a serious infection from them.
- When starting SYMLIN, you will need to reduce your dose of mealtime insulin. Your healthcare provider will tell you how to reduce your dose of mealtime insulin the right way.
- You must check your blood sugar as often as your healthcare provider tells you to, which may include before and after every meal and at bedtime.
- The usual starting dose of SYMLIN for people who have type 1 diabetes is 15 micrograms (mcg) injected under your skin.
- Inject SYMLIN under your skin (subcutaneously) just before a major meal. A major meal must have at least 250 calories or 30 grams of carbohydrate.
- If you have not had any nausea for 3 days or more after your dose of SYMLIN is changed, your healthcare provider may tell you to slowly increase your dose of SYMLIN.
- Tell your healthcare provider right away if you have nausea or low blood sugar after your dose of SYMLIN is changed. Your healthcare provider will tell you what to do.
- Your healthcare provider may make changes to your insulin dose to better control your blood sugar. Your healthcare provider should tell you what the right dose of insulin is for you.
- When starting SYMLIN, you will need to reduce your dose of mealtime insulin. Your healthcare provider will tell you how to reduce your dose of mealtime insulin the right way.
- You must check your blood sugar as often as your healthcare provider advises you to, which may include before and after every meal and at bedtime.
- The usual starting dose of SYMLIN for people who have type 2 diabetes is 60 micrograms (mcg) injected under your skin.
- Inject SYMLIN under your skin (subcutaneously) just before a major meal. A major meal must have at least 250 calories or 30 grams of carbohydrate.
- If you have not had any nausea for 3 days or more after your dose of SYMLIN is changed, your healthcare provider may tell you to increase your dose of SYMLIN.
- Tell your healthcare provider right away if you have nausea or low blood sugar after your dose of SYMLIN is changed. Your healthcare provider will tell you what to do.
- Your healthcare provider may make changes to your insulin dose to better control your blood sugar. Your healthcare provider should tell you what the right dose of insulin is for you.
- See “What is the most important information I should know about SYMLIN?”
- Do not drive, operate machinery, or do other dangerous activities until you know how SYMLIN affects you. Talk to your healthcare provider about the activities you should avoid.
- Alcohol. Drinking alcohol may increase your chances of getting severe low blood sugar.
- See “What is the most important information I should know about SYMLIN?”
- injection site reactions. SYMLIN may cause bruising, swelling, or itching at the injection site.
- nausea
- vomiting
- decreased appetite
- stomach pain
- headache
- Keep SYMLIN in the refrigerator between 36°F to 46°F (2°C to 8°C), until you are ready to use it.
- Do not freeze. Do not use SYMLIN if it has been frozen.
- Keep unopened SYMLIN out of the light.
- Keep SYMLIN either in the refrigerator or at room temperature between 36°F to 86°F (2°C to 30°C) for up to 30 days.
- Throw away used SYMLIN after 30 days of use, even if the pen still has medicine in it.
- Do not use SYMLIN (opened or unopened) after the expiration (EXP) date printed on the carton and the label.