Brand Name
Gynazole 1
Generic Name
Butoconazole
View Brand Information FDA approval date: April 29, 2015
Classification: Azole Antifungal
Form: Cream
What is Gynazole 1 (Butoconazole)?
GYNAZOLE 1 ® Butoconazole Nitrate Vaginal Cream USP, 2% is indicated for the local treatment of vulvovaginal candidiasis . The diagnosis should be confirmed by KOH smears and/or cultures. Note: GYNAZOLE, 1 ® Butoconazole Nitrate Vaginal Cream USP, 2% is safe and effective in non-pregnant women; however, the safety and effectiveness of this product in pregnant women has not been established.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
gynazole 1 (butoconazole nitrate)
1DESCRIPTION
GYNAZOLE • 1

Butoconazole nitrate is a white to off-white crystalline powder with a molecular weight of 474.79. It is sparingly soluble in methanol; slightly soluble in chloroform, methylene chloride, acetone, and ethanol; very slightly soluble in ethyl acetate; and practically insoluble in water. It melts at about 159°C with decomposition.
GYNAZOLE • 1
2CLINICAL PHARMACOLOGY
Following vaginal administration of butoconazole nitrate vaginal cream, 2% to 3 women, 1.7% (range 1.3-2.2%) of the dose was absorbed on average. Peak plasma levels (13.6-18.6 ng radioequivalents/mL of plasma) of the drug and its metabolites are attained between 12 and 24 hours after vaginal administration.
2.1Microbiology -
The exact mechanism of the antifungal action of butoconazole nitrate is unknown; however, it is presumed to function as other imidazole derivatives via inhibition of steroid synthesis. Imidazoles generally inhibit the conversion of lanosterol to ergosterol, resulting in a change in fungal cell membrane lipid composition. This structural change alters cell permeability and, ultimately, results in the osmotic disruption or growth inhibition of the fungal cell.
Butoconazole nitrate is an imidazole derivative that has fungicidal activity
3INDICATIONS AND USAGE
GYNAZOLE • 1
Note: GYNAZOLE • 1® Butoconazole Nitrate Vaginal Cream USP, 2% is safe and effective in non-pregnant women; however, the safety and effectiveness of this product in pregnant women has not been established (see PRECAUTIONS - Pregnancy).
4CONTRAINDICATIONS
GYNAZOLE • 1
5CLINICAL STUDIES
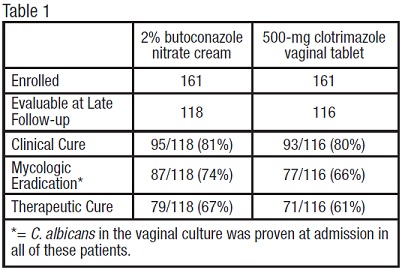
Vulvovaginal Candidiasis: Two studies were conducted that compared 2% butoconazole nitrate cream with clotrimazole tablets. There were 322 enrolled patients, 161 received 2.0% butoconazole vaginal cream and 161 patients inserted the 500-mg clotrimazole vaginal tablet. At the second follow-up visit (30 days post-therapy), 118 patients in the butoconazole group and 116 in the clotrimazole group were evaluable for efficacy analysis, respectively. All of these patients had infection caused by Candida albicans.
The efficacy of the study drugs was assessed by evaluating clinical, mycologic and therapeutic cure rates, which are summarized in Table 1.
The therapeutic cure was defined as complete resolution of signs and symptoms of vaginal candidiasis (clinical cure) along with a negative KOH examination and negative culture for
6WARNINGS
This cream contains mineral oil. Mineral oil may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms; therefore, use of such products within 72 hours following treatment with GYNAZOLE•1
Recurrent vaginal yeast infections, especially those that are difficult to eradicate, can be an early sign of infection with the human immunodeficiency virus (HIV) in women who are considered at risk for HIV infection.
7ADVERSE REACTIONS
Of the 314 patients treated with GYNAZOLE • 1
8DOSAGE AND ADMINISTRATION
The recommended dose of GYNAZOLE • 1
9HOW SUPPLIED
GYNAZOLE•1
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Avoid heat above 30°C (86°F).
10Patient Package Insert
GYNAZOLE•1
Butoconazole Nitrate Vaginal Cream USP,
IN ONE PREFILLED DISPOSABLE APPLICATOR
Using the GYNAZOLE•1
Prefilled Disposable Applicator
3 Easy Steps:
Step 1: Preparing the Applicator
Peel back the protective foil and remove the prefilled applicator. Applicator is designed to be used with tip in place.
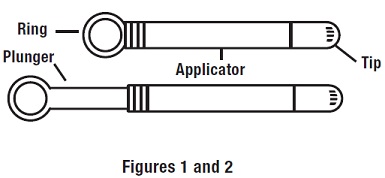
Do not warm applicator before using. While holding the applicator firmly, pull the ring back to fully extend the plunger (see Figures 1 and 2 ).
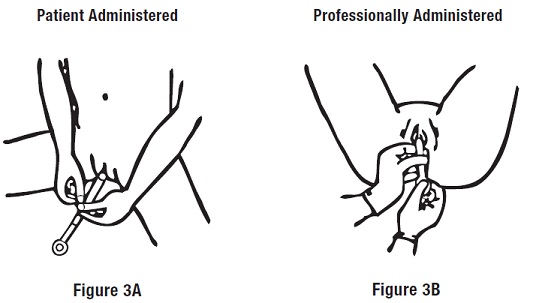
Step 2: Inserting the Applicator
Gently insert the applicator into the vagina as far as it will comfortably go (
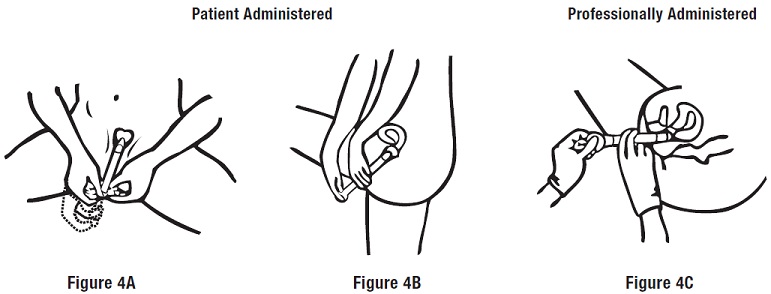
Step 3: Applying the Cream
Push the plunger to release the cream (
Important Instructions
- One prefilled applicator of GYNAZOLE • 1
- This cream contains mineral oil. Mineral oil may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms; therefore, use of such products within 72 hours following treatment with GYNAZOLE • 1
- There are no adequate and well-controlled studies in pregnant women. GYNAZOLE • 1
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
Manufactured By Padagis
Yeruham, Israel
Distributed By
Padagis
Allegan, MI 49010 • www.padagis.com
Rev 05-22
8F200 RC J2
11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rx Only
NDC 45802-
GYNAZOLE•1
Butoconazole Nitrate Vaginal Cream USP, 2%
The applicator delivers approximately 5 g cream, containing 100 mg butoconazole nitrate.
IN ONE PREFILLED DISPOSABLE APPLICATOR
For Vaginal Use Only.
NET WT 5.8 g

The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
