Brand Name
Dapiprazole
View Brand InformationFDA approval date: July 31, 2019
Form: Kit
What is Dapiprazole?
Dapiprazole hydrochloride ophthalmic solution is indicated in the treatment of iatrogenically induced mydriasis produced by adrenergic or parasympatholytic agents. Dapiprazole hydrochloride ophthalmic solution is not indicated for the reduction of intraocular pressure or in the treatment of open angle glaucoma.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
DAPIPRAZOLE (DAPIPRAZOLE)
1DESCRIPTION:
For ophthalmic use only.
Dapiprazole hydrochloride is an alpha-adrenergic blocking agent.
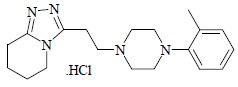
Dapiprazole hydrochloride is 5,6,7,8-tetrahydro-3-[2-(4-
Dapiprazole hydrochloride has the empirical formula C
The structural formula for dapiprazole hydrochloride is:
Dapiprazole hydrochloride is a sterile, white, lyophilized powder soluble in water.
Dapiprazole hydrochloride ophthalmic solution, 0.5% is a clear, colorless, slightly viscous solution for topical application. Each mL (when reconstituted as directed) contains 5 mg of dapiprazole hydrochloride as the active ingredient.
The reconstituted solution has a pH of approximately 6.6 and an osmolarity of approximately 415 mOsm.
The inactive ingredients include: mannitol (2%), sodium chloride, hydroxypropyl methylcellulose (0.4%), edetate sodium (0.01%), sodium phosphate dibasic, sodium phosphate monobasic, water for injection, and benzalkonium chloride (0.01%) as a preservative.
Dapiprazole hydrochloride ophthalmic solution, 0.5% is supplied in a kit consisting of one vial of dapiprazole hydrochloride (25 mg), one vial of diluent (5 mL) and one dropper for dispensing.
2CLINICAL PHARMACOLOGY:
Dapiprazole hydrochloride ophthalmic solution acts through blocking the alpha-adrenergic receptors in smooth muscle. Dapiprazole hydrochloride ophthalmic solution produces miosis through an effect on the dilator muscle of the iris.
Dapiprazole hydrochloride ophthalmic solution does not have any significant activity on ciliary muscle contraction and, therefore does not induce a significant change in the anterior chamber depth or the thickness of the lens.
Dapiprazole hydrochloride ophthalmic solution has demonstrated safe and rapid reversal of mydriasis produced by phenylephrine and to a lesser degree tropicamide. In patients with decreased accommodative amplitude due to treatment with tropicamide, dapiprazole hydrochloride ophthalmic solution partially restores the accommodative amplitude. This activity is not only due to its miotic effect but also to a direct effect on accommodation.
Eye color affects the rate of pupillary constriction. In individuals with brown irides, the rate of pupillary constriction may be slightly slower than in individuals with blue or green irides. Eye color does not appear to affect the final pupil size.
Dapiprazole hydrochloride ophthalmic solution does not significantly alter intraocular pressure in normotensive eyes or in eyes with elevated intraocular pressure.
3INDICATIONS AND USAGE:
Dapiprazole hydrochloride ophthalmic solution is indicated in the treatment of iatrogenically induced mydriasis produced by adrenergic (phenylephrine) or parasympatholytic (tropicamide) agents. Dapiprazole hydrochloride ophthalmic solution is not indicated for the reduction of intraocular pressure or in the treatment of open angle glaucoma.
4CONTRAINDICATIONS:
Miotics are contraindicated where constriction is undesirable; such as acute iritis, and in those subjects showing hypersensitivity to any component of this preparation.
5WARNING:
For Topical Ophthalmic Use Only. NOT FOR INJECTION. Do not touch the dropper up to lids or any surface, as this may contaminate the solution. Dapiprazole hydrochloride ophthalmic solution should not be used in the same patient more frequently than once a week.
6ADVERSE REACTIONS:
In controlled studies the most frequent reaction to dapiprazole was conjunctival injection lasting 20 minutes in over 80% of patients. Burning on instillation of dapiprazole hydrochloride ophthalmic solution was reported in approximately half of all patients. Reactions occurring in 10% to 40% of patients included ptosis, lid erythema, lid edema, chemosis, itching, punctate keratitis, corneal edema, browache, photophobia and headaches. Other reactions reported less frequently included dryness of eyes, tearing and blurring of vision.
7DOSAGE AND ADMINISTRATION:
Two drops followed 5 minutes later by an additional 2 drops applied topically to the conjunctiva of each eye should be administered after the ophthalmic examination to reverse the diagnostic mydriasis. Dapiprazole hydrochloride ophthalmic solution should not be used in the same patient more frequently than once per week.
7.1Directions for Preparing Eyedrops:
- Use aseptic technique.
- Tear off aluminum seals, remove and discard rubber plugs from both drug and diluent vials
- Pour diluent into drug vial.
- Remove dropper assembly from its sterile wrapping and attach to the drug vial.
- Shake container for several minutes to ensure mixing.
8HOW SUPPLIED:
Dapiprazole hydrochloride ophthalmic solution, 0.5% - Sterile is supplied as an outer package (NDC 53020-265-01) containing:
NDC 53020-255-01 single vial of dapiprazole hydrochloride (25 mg) lyophilized powder
NDC 53020-245-01 single vial of diluent (5 mL)
Single dropper for dispensing
9Storage and Stability of Eyedrops:
Once the ophthalmic solution has been reconstituted it may be stored at 20° - 25°C (68° - 77°F) [See USP Controlled Temperature] for 21 days. Discard any solution that is not clear and colorless.
Updated: February 2019
Manufactured by:
Exela Pharma Sciences, LLC
Lenoir, NC 28645
Exela Pharma Sciences, LLC
Lenoir, NC 28645
Manufactured For:
Baradaina, LLC
8780 W. Golf Road, Suite 304
Niles , Illinois 60714
Baradaina, LLC
8780 W. Golf Road, Suite 304
Niles , Illinois 60714
Rx only
10PRINCIPAL DISPLAY-Container Label
Sterile
Dapiprazole HCI
Ophthalmic Solution
0.5% E Y E D R O P S
Rx only
For Use in the Eyes
BARADAINA, LLC
