Brand Name
Elmiron
Generic Name
Polysulfate
View Brand Information FDA approval date: September 26, 1996
Classification: Glycosaminoglycan
Form: Capsule
What is Elmiron (Polysulfate)?
ELMIRON ® is indicated for the relief of bladder pain or discomfort associated with interstitial cystitis.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 3, Randomised, Double-Blind, Placebo-Controlled Multi-Centre Study to Evaluate the Treatment Effect of Pentosan Polysulfate Sodium Compared to Placebo in Participants With Knee Osteoarthritis Pain
Summary: The purpose of this study is to measure the change in pain and function with subcutaneous injections of pentosan polysulfate sodium (PPS) compared with subcutaneous injections of placebo in participants with knee OA pain. Study details include: * The study duration will be up to 64 weeks. * The treatment duration will be 6 weeks. * The visit frequency will be twice weekly during treatment. * The v...
A Randomized, Controlled, Phase II/III Trial to Evaluate the Efficacy and Safety of U101 Oral Capsules in Radiation-induced Cystitis
Summary: The purpose of this study is to evaluate the efficacy and safety of pentosan polysulfate sodium versus placebo and in patients with radiation cystitis who have received radiation therapy in pelvic region.
Related Latest Advances
Brand Information
ELMIRON (PENTOSAN POLYSULFATE SODIUM)
1DESCRIPTION
Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative, which chemically and structurally resembles glycosaminoglycans. It is a white odorless powder, slightly hygroscopic and soluble in water to 50% at pH 6. It has a molecular weight of 4000 to 6000 Dalton with the following structural formula:
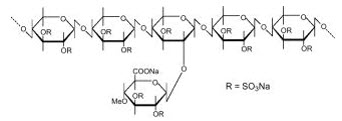
ELMIRON
2CLINICAL TRIALS
ELMIRON
A second clinical trial, the physician's usage study, was a prospectively designed retrospective analysis of 2499 patients who received ELMIRON
Patients had unblinded evaluations every 3 months for the patient's rating of overall change in pain in comparison to baseline and for the difference calculated in "pain/discomfort" scores. At baseline, pain/discomfort scores for the original 2499 patients were severe or unbearable in 60%, moderate in 33% and mild or none in 7% of patients. The extent of the patients' pain improvement is shown in
At 3 months, 722/2499 (29%) of the patients originally in the study had pain scores that improved by one or two categories. By 6 months, in the 892 patients who continued taking ELMIRON
3INDICATIONS AND USAGE
ELMIRON
4CONTRAINDICATIONS
ELMIRON
5WARNINGS
Retinal Pigmentary Changes
Pigmentary changes in the retina, reported in the literature as pigmentary maculopathy, have been identified with long-term use of ELMIRON
6ADVERSE REACTIONS
ELMIRON
Deaths occurred in 6/2627 (0.2%) patients who received the drug over a period of 3 to 75 months. The deaths appear to be related to other concurrent illnesses or procedures, except in one patient for whom the cause was not known.
Serious adverse events occurred in 33/2627 (1.3%) patients. Two patients had severe abdominal pain or diarrhea and dehydration that required hospitalization. Because there was not a control group of patients with interstitial cystitis who were concurrently evaluated, it is difficult to determine which events are associated with ELMIRON
The adverse events described below were reported in an unblinded clinical trial of 2499 interstitial cystitis patients treated with ELMIRON
Frequency (1 to 4%): Alopecia (4%), diarrhea (4%), nausea (4%), headache (3%), rash (3%), dyspepsia (2%), abdominal pain (2%), liver function abnormalities (1%), dizziness (1%).
Frequency (≤ 1%):
Digestive:Vomiting, mouth ulcer, colitis, esophagitis, gastritis, flatulence, constipation, anorexia, gum hemorrhage.
Hematologic:Anemia, ecchymosis, increased prothrombin time, increased partial thromboplastin time, leukopenia, thrombocytopenia.
Hypersensitive Reactions:Allergic reaction, photosensitivity.
Respiratory System:Pharyngitis, rhinitis, epistaxis, dyspnea.
Skin and Appendages:Pruritus, urticaria.
Special Senses:Conjunctivitis, tinnitus, optic neuritis, amblyopia, retinal hemorrhage.
6.1Post-Marketing Experience
The following adverse reactions have been identified during post approval use of pentosan polysulfate sodium; because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- pigmentary changes in the retina (see
6.1.1Rectal Hemorrhage
ELMIRON
6.1.2Liver Function Abnormality
A randomized, double-blind, parallel group, Phase 2 study was conducted in 100 men (51 ELMIRON
7OVERDOSAGE
Overdose has not been reported. Based upon the pharmacodynamics of the drug, toxicity is likely to be reflected as anticoagulation, bleeding, thrombocytopenia, liver function abnormalities, and gastric distress (see
8DOSAGE AND ADMINISTRATION
The recommended dose of ELMIRON
Patients receiving ELMIRON
The clinical value and risks of continued treatment in patients whose pain has not improved by 6 months is not known.
9HOW SUPPLIED
ELMIRON
NDC NUMBER 50458-098-01
9.1Storage
Store at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted to 15 °C to 30 °C (59 °F to 86 °F).
Keep out of reach of children.
10PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
NDC 50458-098-01
ELMIRON
CAPSULES
100 mg
Attention: Dispense the enclosed
Each capsule contains 100 mg
Rx only
100 Capsules
janssen



