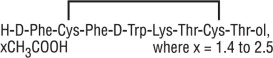Octreotide Acetate
What is Octreotide (Octreotide Acetate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of the current study is to evaluate the efficacy and safety of \[177Lu\]Lu-DOTA-TATE plus octreotide long-acting release (LAR) versus octreotide LAR alone in newly diagnosed patients with somatostatin receptor positive (SSTR+), well differentiated Grade1 and Grade 2 (G1 and G2) (Ki-67 \<10%) advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs) with high disease burden
Summary: This study aims to determine the safety, pharmacokinetics (PK) and recommended Phase 3 dose (RP3D) of RYZ101 in Part 1, and the safety, efficacy, and PK of RYZ101 compared with investigator-selected standard of care (SoC) therapy in Part 2 in subjects with inoperable, advanced, well-differentiated, somatostatin receptor expressing (SSTR+) gastroenteropancreatic neuroendocrine tumors (GEP-NETs) tha...
Summary: Between 10% and 15% of patients with endogenous hypercortisolism (Cushing syndrome) have ectopic (non-pituitary) production of adrenocorticotropin hormone (ACTH) that causes cortisol excess. In approximately 50% of these patients, the tumoral source of ACTH cannot be found initially despite very detailed and extensive imaging, including studies such as computed tomography, magnetic resonance imagi...
Related Latest Advances
Brand Information
- sodium chloride…………………..……... 7 mg
- glacial acetic acid, USP ………………… 2 mg
- sodium acetate trihydrate, USP ………… 2 mg
- water for injection, USP ……………qs to 1 mL