Fasenra
What is Fasenra (Benralizumab)?
Approved To Treat
Related Clinical Trials
Summary: A study to evaluate the efficacy and safety of benralizumab administered subcutaneously in patients ≥ 6 to \< 18 years of age with severe eosinophilic asthma, including a well-documented history of asthma exacerbations and uncontrolled asthma receiving high-dose inhaled corticosteroid (ICS) plus at least one additional controller medication.
Summary: The objective of this study is to collect empirical data that elucidates the clinical profile and therapeutic efficacy of benralizumab among the patients aged 12 years and above, with severe eosinophilic asthma. The study will focus on the early treatment response, treatment outcomes and the change in asthma control of benralizumab therapy in a real-world setting in China. This study will also des...
Summary: The main purpose of study is to assess the safety, tolerability, and pharmacokinetic (PK) of benralizumab.
Related Latest Advances
Brand Information
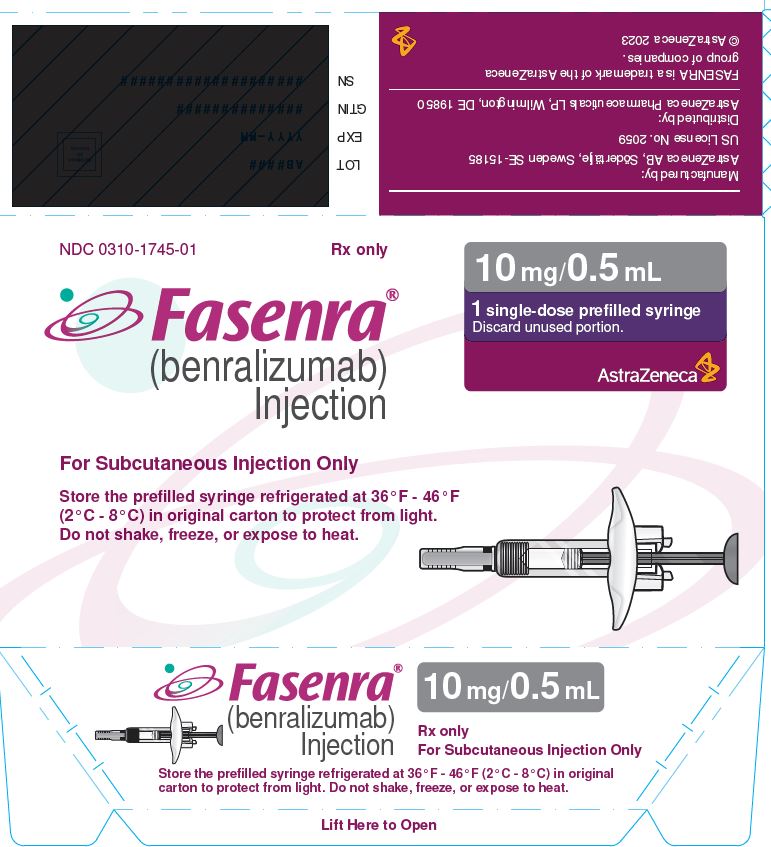
- 10 mg/0.5 mL solution in a single-dose prefilled syringe.
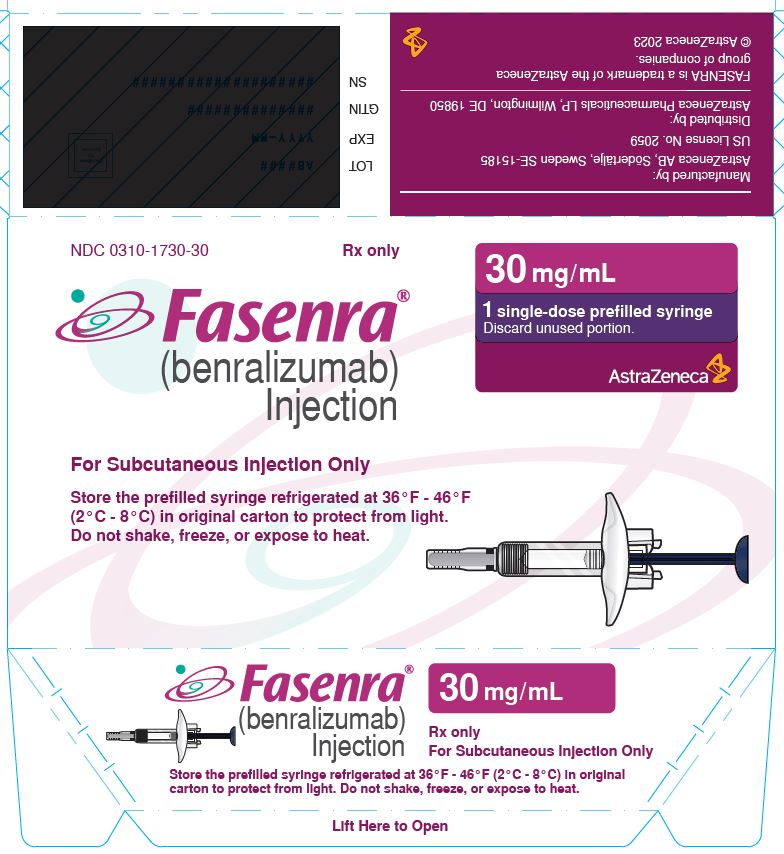
- 30 mg/mL solution in a single-dose prefilled syringe.
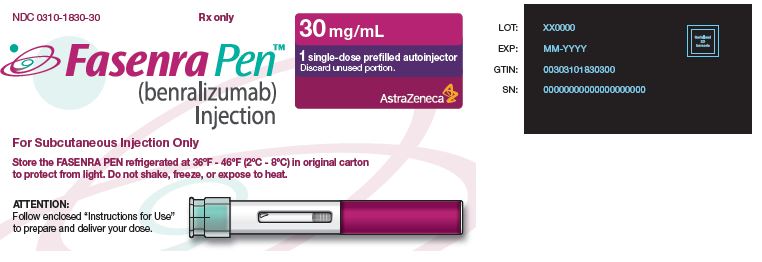
- 30 mg/mL solution in a single-dose autoinjector FASENRA PEN.
- Hypersensitivity Reactions
- Carton contains one 10 mg/0.5 mL single-dose prefilled syringe with a gray plunger rod: NDC 0310-1745-01
- Carton contains one 30 mg/mL single-dose prefilled syringe with a blue plunger rod: NDC 0310-1730-30
- Carton contains one 30 mg/mL single-dose autoinjector: NDC 0310-1830-30