Ubrelvy
What is Ubrelvy (Ubrogepant)?
For millions of people living with migraines, an attack can feel like losing control of the day, intense head pain, light sensitivity, and nausea that stop everything in its tracks. For those who can’t take or don’t respond well to traditional migraine medications like triptans, newer therapies offer hope. Ubrelvy (generic name: ubrogepant) is one such treatment designed to stop migraine attacks once they start, helping patients regain comfort and functionality faster.
Ubrelvy belongs to a newer class of medications known as CGRP receptor antagonists. It’s the first oral medication in this group approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine, with or without aura, in adults. Unlike preventive migraine drugs, Ubrelvy is used on demand, when a migraine begins, offering a modern, targeted way to manage symptoms and reduce the disabling impact of attacks.
What does Ubrelvy do?
Ubrelvy is prescribed to treat migraine attacks after they start, not to prevent future ones. It works by relieving the pain and associated symptoms such as nausea, sensitivity to light (photophobia), and sound (phonophobia).
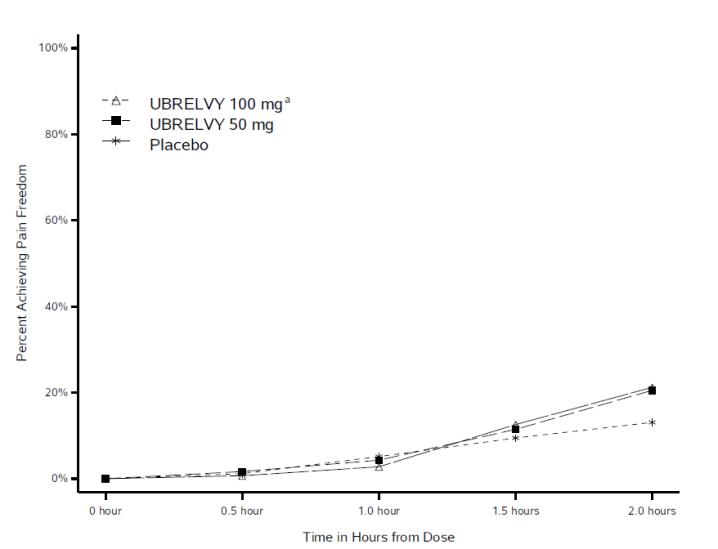
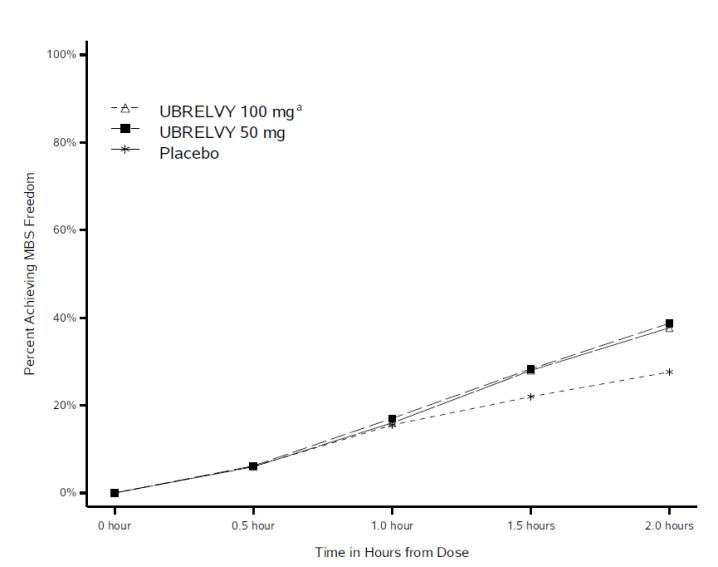
In clinical trials, many patients reported meaningful pain relief within two hours of taking Ubrelvy, with some experiencing sustained relief for up to 24 hours (FDA, 2019). Unlike triptans, which constrict blood vessels, Ubrelvy works through a different biological pathway, making it an alternative for patients who cannot use triptans due to cardiovascular conditions.
By quickly blocking migraine signals in the brain, Ubrelvy allows patients to resume normal activities sooner, which can significantly improve quality of life. It is often considered a first-line oral option for patients seeking effective, well-tolerated, and non-vasoconstrictive migraine relief.
How does Ubrelvy work?
Migraines are believed to result from the overactivation of pain pathways in the brain involving a molecule called calcitonin gene-related peptide (CGRP). During an attack, CGRP levels rise, causing inflammation and widening of blood vessels, which contribute to throbbing pain and sensitivity.
Ubrelvy targets this mechanism directly by blocking CGRP receptors. When these receptors are blocked, CGRP cannot attach to them, effectively interrupting the migraine process and reducing pain transmission.
Clinically, this mechanism is important because it stops migraine progression without causing blood vessel constriction, making it safer for patients with a history of heart disease, stroke, or uncontrolled hypertension, conditions where triptans are often contraindicated.
In short, Ubrelvy doesn’t just mask the pain; it works at the molecular level to halt the biological process driving the migraine itself.
Ubrelvy side effects
Ubrelvy is generally well tolerated, but as with any medication, side effects can occur. Most are mild and temporary.
Common side effects include:
- Nausea
- Sleepiness or fatigue
- Dry mouth
Less common side effects:
- Dizziness
- Mild changes in taste
- Abdominal discomfort
Serious but rare side effects:
- Severe allergic reactions (rash, itching, or swelling of the face or throat)
- Shortness of breath or chest tightness
- Liver-related issues in people taking other medications that affect liver enzymes
Patients should seek immediate medical attention if they experience symptoms of an allergic reaction or jaundice (yellowing of skin or eyes).
Those with severe liver or kidney disease should discuss alternative options with their doctor, as Ubrelvy’s clearance depends partly on these organs. Patients taking medications that strongly affect liver enzymes (CYP3A4 inhibitors or inducers) may also require dose adjustments or should avoid concurrent use (Mayo Clinic, 2024).
Overall, clinical data show that most side effects are mild and self-limiting, and the drug is considered safe for repeated use as directed by a physician.
Ubrelvy dosage
Ubrelvy comes as an oral tablet taken as soon as migraine symptoms begin. It can be taken with or without food, and patients may take a second dose after a few hours if symptoms persist, following their doctor’s specific instructions.
While no routine blood testing is typically required, monitoring may be advised for patients with:
- Liver disease, to assess how well the body processes the drug
- Kidney impairment, to ensure proper clearance
- Polypharmacy (multiple medications), especially if other drugs affect CYP3A4 metabolism
Doctors often remind patients not to exceed the recommended dosing frequency to prevent potential overuse headaches or increased side effects.
For older adults, no specific dosage changes are usually needed, but careful monitoring ensures that drug interactions or organ function issues are identified early.
Does Ubrelvy have a generic version?
As of 2025, no FDA-approved generic version of Ubrelvy is available. The medication is currently manufactured and distributed by AbbVie Inc., which markets it under the brand name Ubrelvy.
Because it’s a relatively new therapy (approved in 2019), patent protections are still active, and a generic form is not expected for several years.
Patients concerned about affordability can explore manufacturer savings programs or insurance coverage options, as AbbVie offers copay assistance for eligible individuals. When a generic version becomes available, it will be required to meet FDA standards ensuring it is bioequivalent, equally effective, and safe as the brand-name drug.
Conclusion
Ubrelvy represents a major step forward in migraine care, offering targeted, effective relief without the vascular risks associated with older migraine drugs. For patients who experience frequent or severe attacks, this medication can mean faster recovery, less disruption to daily life, and renewed confidence in managing their condition.
Though not every patient responds the same way, consistent communication with a healthcare provider helps ensure the best treatment outcomes. Doctors can tailor Ubrelvy use based on attack frequency, other medications, and overall health profile.
When prescribed and monitored by a qualified healthcare professional, Ubrelvy is a safe and effective way to stop migraines in their tracks, helping patients reclaim comfort, clarity, and control when they need it most.
References
- U.S. Food and Drug Administration (FDA). Ubrelvy (ubrogepant) Prescribing Information. 2019. https://www.fda.gov/
- Mayo Clinic. “Ubrogepant (Oral Route).” Updated 2024. https://www.mayoclinic.org/
- National Institutes of Health (NIH). MedlinePlus: Ubrogepant Oral Tablets. Accessed 2024. https://www.nih.gov/
Top Global Experts
Related Clinical Trials
Summary: Migraine is a common neurological disorder typically characterized by attacks of throbbing or pulsating headache on one side of the head of moderate to severe pain intensity. The purpose of this study is to evaluate fetal, maternal, and infant outcomes through 12 months of age among women exposed to Ubrelvy or Qulipta during pregnancy, as well as in 2 comparator groups. Ubrelvy (ubrogepant) and Qu...
Summary: A migraine is a moderate to severe headache typically on one side of the head. A migraine attack is a headache that may be accompanied by throbbing, nausea, vomiting, sensitivity to light and sound, or other symptoms. Menstrual migraine (MM) is defined as migraine attacks that occur within the perimenstrual period (PMP) in at least 2 out of 3 menstrual cycles. The PMP is from 2 days before the ons...
Summary: Migraine is a common neurological disorder typically characterized by attacks of throbbing, moderate to severe headache, often associated with nausea, vomiting, and sensitivity to light and sound. Migraine is extremely common and disabling in children. The purpose of this study is to evaluate how safe and effective ubrogepant is in the acute treatment of migraine in children and adolescents. Ubrog...
Related Latest Advances
Brand Information
- With concomitant use of strong CYP3A4 inhibitors
- In patients with a history of serious hypersensitivity to ubrogepant or any component of UBRELVY. Reactions have included anaphylaxis, dyspnea, and facial or throat edema
- Hypersensitivity Reactions
- Hypertension
- Raynaud’s Phenomenon
![The following structural formula for UBRELVY is ubrogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist. The chemical name of ubrogepant is (3'S)-N-((3S,5S,6R)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=ubrelvy-01.jpg&setid=fd9f9458-fd96-4688-be3f-f77b3d1af6ab)
contains
10 tablets
Rx Only
UBRELVY®
(ubrogepant) tablets
50 mg

contains
10 tablets
Rx Only
UBRELVY®
(ubrogepant) tablets
100 mg

