Generic Name
Oxaprozin
Brand Names
Daypro, Coxanto
FDA approval date: October 29, 1992
Classification: Nonsteroidal Anti-inflammatory Drug
Form: Tablet, Capsule
What is Daypro (Oxaprozin)?
Oxaprozin tablets are indicated: For relief of the signs and symptoms of osteoarthritis, For relief of the signs and symptoms of rheumatoid arthritis, For relief of the signs and symptoms of juvenile rheumatoid arthritis Oxaprozin tablets are a non-steroidal anti-inflammatory drug indicated for:, Relief of signs and symptoms of Osteoarthritis , Relief of signs and symptoms of Rheumatoid Arthritis , Relief of signs and symptoms of Juvenile Rheumatoid Arthritis
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Daypro (oxaprozin)
1INDICATIONS AND USAGE
DAYPRO is indicated:
- For relief of the signs and symptoms of osteoarthritis
- For relief of the signs and symptoms of rheumatoid arthritis
- For relief of the signs and symptoms of juvenile rheumatoid arthritis
2DOSAGE FORMS AND STRENGTHS
DAYPRO (oxaprozin) caplets: 600 mg caplets, white, capsule-shaped, scored, film-coated, with DAYPRO debossed on one side and 1381 on the other side.
3CONTRAINDICATIONS
DAYPRO is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to oxaprozin or any components of the drug product [
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [
- In the setting of CABG surgery [
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events [
- GI Bleeding, Ulceration and Perforation [
- Hepatotoxicity [
- Hypertension [
- Heart Failure and Edema [
- Renal Toxicity and Hyperkalemia [
- Anaphylactic Reactions [
- Serious Skin Reactions [
- Hematologic Toxicity [
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reaction data were derived from patients who received DAYPRO in multidose, controlled, and open-label clinical trials. Rates for events from clinical trial experience are based on 2253 patients who took 1200 mg to 1800 mg DAYPRO per day in clinical trials. Of these, 1721 patients were treated for at least 1 month, 971 patients for at least 3 months, and 366 patients for more than 1 year.
Incidence Greater than 1%: In clinical trials of DAYPRO or in patients taking other NSAIDs, the following adverse reactions occurred at an incidence greater than 1%.
Cardiovascular system: edema.
Digestive system: abdominal pain/distress, anorexia, constipation, diarrhea, dyspepsia, flatulence, gastrointestinal ulcers (gastric/duodenal), gross bleeding/perforation, heartburn, liver enzyme elevations, nausea, vomiting.
Hematologic system: anemia, increased bleeding time.
Nervous system: CNS inhibition (depression, sedation, somnolence, or confusion), disturbance of sleep, dizziness, headache.
Skin and appendages: pruritus, rash.
Special senses: tinnitus.
Urogenital system: abnormal renal function, dysuria or frequency.
Incidence Greater than 1%: The following adverse reactions were reported in clinical trials or in patients taking other NSAIDs.
Body as a whole: appetite changes, death, drug hypersensitivity reactions including anaphylaxis, fever, infection, sepsis.
Cardiovascular system: arrhythmia, blood pressure changes, congestive heart failure, hypertension, hypotension, myocardial infarction, palpitations, tachycardia, syncope, vasculitis.
Digestive system: alteration in taste, dry mouth, eructation, esophagitis, gastritis, glossitis, hematemesis, jaundice, liver function abnormalities including liver failure, stomatitis, hemorrhoidal or rectal bleeding.
Hematologic system: aplastic anemia, ecchymoses, eosinophilia, hemolytic anemia, lymphadenopathy, melena, purpura, thrombocytopenia, leukopenia.
Metabolic system: hyperglycemia, weight changes.
Nervous system: anxiety, asthenia, coma, convulsions, dream abnormalities, drowsiness, hallucinations, insomnia, malaise, meningitis, nervousness, paresthesia, tremors, vertigo, weakness.
Respiratory system: asthma, dyspnea, pulmonary infections, pneumonia, sinusitis, symptoms of upper respiratory tract infection, respiratory depression.
Skin: alopecia, angioedema, urticaria, photosensitivity, sweat.
Special senses: blurred vision, conjunctivitis, hearing decrease.
Urogenital: cystitis, hematuria, increase in menstrual flow, oliguria/ polyuria, proteinuria, renal insufficiency, decreased menstrual flow.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of DAYPRO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a whole: serum sickness.
Digestive system: hepatitis, pancreatitis.
Hematologic system: agranulocytosis, pancytopenia.
Skin: pseudoporphyria, exfoliative dermatitis, erythema multiforme, Stevens-Johnson Syndrome, fixed drug eruption (FDE), toxic epidermal necrolysis (Lyell's syndrome).
Urogenital: acute interstitial nephritis, nephrotic syndrome, acute renal failure.
5DRUG INTERACTIONS
See
6OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [
Manage patients with symptomatic and supportive care following an acute NSAID overdosage. There are no specific antidotes. It is advisable to contact a poison control center (1-800-222-1222) to determine the latest recommendations because strategies for the management of overdose are continually evolving.
If gastric decontamination may be potentially beneficial to the patient, e.g., short time since ingestion or a large overdosage (5 to 10 times the recommended dosage), consider emesis and/or activated charcoal (60 grams to 100 grams in adults, 1 gram to 2 grams per kg of body weight in pediatric patients) and/or an osmotic cathartic in symptomatic patients. Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
7DESCRIPTION
DAYPRO (oxaprozin) caplet is a nonsteroidal anti-inflammatory drug, available as caplets of 600 mg for oral administration. The chemical name is 4,5-diphenyl-2-oxazole-propionic acid. The molecular weight is 293. Its molecular formula is C
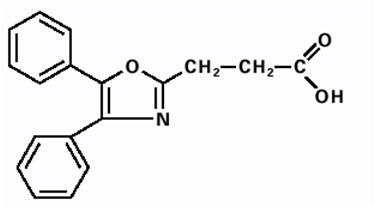
Oxaprozin is a white to off-white powder with a slight odor and a melting point of 162°C to 163°C. It is slightly soluble in alcohol and insoluble in water, with an octanol/water partition coefficient of 4.8 at physiologic pH (7.4). The pK
The inactive ingredients in DAYPRO include: microcrystalline cellulose, hypromellose, methylcellulose, magnesium stearate, polacrilin potassium, starch, polyethylene glycol and titanium dioxide. DAYPRO 600-mg caplets are white, capsule-shaped, scored, film-coated, with DAYPRO debossed on one side and 1381 on the other side.
8HOW SUPPLIED/STORAGE AND HANDLING
DAYPRO (oxaprozin) 600 mg caplets are white, capsule-shaped, scored, film-coated, with DAYPRO debossed on one side and 1381 on the other side, supplied as:
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with DAYPRO and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, instruct patients to stop DAYPRO and seek immediate medical therapy [
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [
Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur [
Serious Skin Reactions, including DRESS
Advise patients to stop taking DAYPRO immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible [
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including DAYPRO, may be associated with a reversible delay in ovulation [
Fetal Toxicity
Inform pregnant women to avoid use of DAYPRO and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with DAYPRO is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of DAYPRO with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy [
Use of NSAIDS and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with DAYPRO until they talk to their healthcare provider [
10PRINCIPAL DISPLAY PANEL - 600 mg Caplet Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0025-1381-31
Pfizer
Daypro
600 mg
100 Caplets
