Brand Name
Cardene
Generic Name
Nicardipine
View Brand Information FDA approval date: January 30, 1992
Classification: Dihydropyridine Calcium Channel Blocker
Form: Injection, Capsule
What is Cardene (Nicardipine)?
Cardene I.V. Premixed Injection is a calcium channel blocker indicated for the short-term treatment of hypertension when oral therapy is not feasible.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Cardene IV (nicardipine hydrochloride)
1DOSAGE FORMS AND STRENGTHS
Injection: 200 mL nicardipine (0.1 mg/mL) in either dextrose (4.8%) or sodium chloride (0.86%) as a clear, colorless solution, ready-to-use, iso-osmotic solution in a single-dose GALAXY container
Injection: 200 mL nicardipine (0.2 mg/mL) in sodium chloride (0.83%) as a clear, colorless solution, ready-to-use, iso-osmotic solution in a single-dose GALAXY container
2OVERDOSAGE
Several overdosages with orally administered nicardipine have been reported. One adult patient allegedly ingested 600 mg of immediate-release oral nicardipine, and another patient, 2160 mg of the sustained-release formulation of nicardipine. Symptoms included marked hypotension, bradycardia, palpitations, flushing, drowsiness, confusion and slurred speech. All symptoms resolved without sequelae. An overdosage occurred in a one year old child who ingested half of the powder in a 30 mg nicardipine standard capsule. The child remained asymptomatic.
Based on results obtained in laboratory animals, lethal overdose may cause systemic hypotension, bradycardia (following initial tachycardia) and progressive atrioventricular conduction block. Reversible hepatic function abnormalities and sporadic focal hepatic necrosis were noted in some animal species receiving very large doses of nicardipine.
For treatment of overdosage, implement standard measures including monitoring of cardiac and respiratory functions. Position the patient so as to avoid cerebral anoxia. Use vasopressors for patients exhibiting profound hypotension.
3DESCRIPTION
CARDENE I.V. (nicardipine hydrochloride) is a calcium ion influx inhibitor (slow channel blocker or calcium channel blocker). CARDENE I.V. for intravenous administration contains 20 mg (0.1 mg/mL) of nicardipine hydrochloride per 200 mL in either dextrose or sodium chloride or 40 mg (0.2 mg/mL) of nicardipine hydrochloride per 200 mL in sodium chloride. Nicardipine hydrochloride is a dihydropyridine derivative with IUPAC (International Union of Pure and Applied Chemistry) chemical name (±)-2-(benzyl-methyl amino) ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate monohydrochloride and has the following structure:
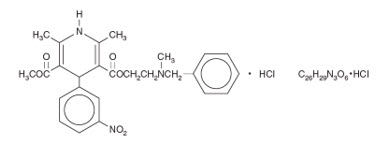
Nicardipine hydrochloride is a greenish-yellow, odorless, crystalline powder that melts at about 169
CARDENE I.V. is available as a ready-to-use sterile, non-pyrogenic, clear, colorless to yellow, iso-osmotic solution for intravenous administration in a 200 mL GALAXY container with 20 mg (0.1 mg/mL) or 40 mg (0.2 mg/mL) nicardipine hydrochloride in either dextrose or sodium chloride.
Nicardipine Hydrochloride in 4.8% Dextrose Injection
20 mg in 200 mL (0.1 mg/mL)
Each mL contains 0.1 mg nicardipine hydrochloride, 48 mg dextrose hydrous, USP, 0.0192 mg citric acid, anhydrous, USP, and 1.92 mg sorbitol, NF. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 3.7 to 4.7.
Nicardipine Hydrochloride in 0.86% Sodium Chloride Injection
20 mg in 200 mL (0.1 mg/mL)
Each mL contains 0.1 mg nicardipine hydrochloride, 8.6 mg sodium chloride, USP, 0.0192 mg citric acid, anhydrous, USP, and 1.92 mg sorbitol, NF. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 3.7 to 4.7.
Nicardipine Hydrochloride in 0.83% Sodium Chloride Injection
40 mg in 200 mL (0.2 mg/mL)
Each mL contains 0.2 mg nicardipine hydrochloride, 8.3 mg sodium chloride, USP, 0.0384 mg citric acid, anhydrous, USP, and 3.84 mg sorbitol, NF. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 3.7 to 4.7.
The GALAXY container is fabricated from multilayered plastic. Solutions are in contact with the polyethylene layer of the container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability and safety of the plastic have been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
4CLINICAL STUDIES
Effects In Hypertension
In patients with mild to moderate chronic stable essential hypertension, CARDENE I.V. (0.5 to 4 mg/hr) produced dose-dependent decreases in blood pressure. At the end of a 48-hour infusion at 4 mg/hr, the decreases were 26 mmHg (17%) in systolic blood pressure and 20.7 mmHg (20%) in diastolic blood pressure. In other settings (e.g., patients with severe or postoperative hypertension), CARDENE I.V. (5 to 15 mg/hr) produced dose-dependent decreases in blood pressure. Higher infusion rates produced therapeutic responses more rapidly. The mean time to therapeutic response for severe hypertension, defined as diastolic blood pressure ≤95 mmHg or ≥25 mmHg decrease and systolic blood pressure ≤160 mmHg, was 77 ± 5.2 minutes. The average maintenance dose was 8 mg/hr. The mean time to therapeutic response for postoperative hypertension, defined as ≥15% reduction in diastolic or systolic blood pressure, was 11.5 ± 0.8 minutes. The average maintenance dose was 3 mg/hr.
5PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

CARDENE I.V.
Nicardipine Hydrochloride
in 0.86% Sodium Chloride Injection
Nicardipine Hydrochloride
in 0.86% Sodium Chloride Injection
For Intravenous Infusion Only.
20 mg in 200 mL
Galaxy
200 mLIso-osmotic
NDC 43066-021-10
Rx only
Sterile, Nonpyrogenic
Rx only
Sterile, Nonpyrogenic
Each mL contains 0.1 mg NICARDIPINE HYDROCHLORIDE in 8.6 mg SODIUM
DOSAGE: See prescribing information.
CAUTIONS: Check for minute leaks by squeezing bag firmly. If leaks are
found, discard bag as sterility may be impaired. Do not use unless solution
is clear. Do not add supplemental medication. Must not be used in series
connections.
found, discard bag as sterility may be impaired. Do not use unless solution
is clear. Do not add supplemental medication. Must not be used in series
connections.
STORAGE: Store at controlled room temperature 20º to 25º C (68º to 77º F);
U.S. Patent Numbers 7612102 and 7659291
Code 2G3445
Novaplus Logo
Manufactured and Marketed by:
To report suspected adverse reactions, contact Baxter Healthcare Corporation
07-34-00-2441
BAR CODE


CARDENE I.V.Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
20mg in 200 mL
NDC 43066-021-10 Rx only
CARDENE I.V.Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
For Intravenous Infusion Only.
Novaplus
Code 2G3445
20 mg in 200 mL
STORE IN CARTON
1 Galaxy Single Dose Container
CARDENE I.V.Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
20 mg in 200 mL
CARDENE I.V.Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
20 mg in 200 mL
BAR CODE
Iso-osmotic Sterile, nonpyrogenic
Each mL contains 0.1 mg NICARDIPINE HYDROCHLORIDE in 8.6 mg SODIUM CHLORIDE, USP with
DOSAGE: See prescribing information.
CAUTIONS: Check for minute leaks by squeezing bag firmly. If leaks are found, discard bag
as sterility may be impaired. Do not use unless solution is clear. Do not add
supplemental medication. Must not be used in series connections.
as sterility may be impaired. Do not use unless solution is clear. Do not add
supplemental medication. Must not be used in series connections.
STORAGE: Store at controlled room temperature 20º to 25º C (68º to 77º F); refer to USP
Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
U.S. Patent Numbers 7612102 and 7659291
Manufactured and Marketed by:
To report suspected adverse reactions, contact Baxter Healthcare Corporation
07-01-00-1496
CARDENE I.V.Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
20 mg in 200 mL
CARDENE I.V.
Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection
20 mg in 200 mL


CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
For Intravenous Infusion Only.
40 mg in 200 mL
Galaxy
200 mLIso-osmotic
NDC 43066-024-10
Rx only
Sterile, Nonpyrogenic
Rx only
Sterile, Nonpyrogenic
Each mL contains 0.2 mg NICARDIPINE HYDROCHLORIDE in 8.3 mg SODIUM
DOSAGE: See prescribing information.
CAUTIONS: Check for minute leaks by squeezing bag firmly. If leaks are
found, discard bag as sterility may be impaired. Do not use unless solution
is clear. Do not add supplemental medication. Must not be used in series
connections.
found, discard bag as sterility may be impaired. Do not use unless solution
is clear. Do not add supplemental medication. Must not be used in series
connections.
STORAGE: Store at controlled room temperature 20º to 25º C (68º to 77º F); refer
to USP Controlled Room Temperature. Protect from freezing. Avoid excessive
heat. PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
to USP Controlled Room Temperature. Protect from freezing. Avoid excessive
heat. PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
U.S. Patent Numbers 7612102 and 7659291
Code 2G3446
Novaplus
Manufactured and Marketed by:
To report suspected adverse reactions, contact Baxter Healthcare Corporation
07-34-00-2442
BAR CODE


CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
40mg in 200 mL
NDC 43066-024-10 Rx only
CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
For Intravenous Infusion Only.
DOUBLE CONCENTRATION:
Novaplus
Code 2G3446
40 mg in 200 mL
STORE IN CARTON
1 Galaxy Single Dose Container
CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
40 mg in 200 mL
DOUBLE CONCENTRATION:
CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
40 mg in 200 mL
BAR CODE
Iso-osmotic Sterile, nonpyrogenic
Each mL contains 0.2 mg NICARDIPINE HYDROCHLORIDE in 8.3 mg SODIUM CHLORIDE, USP
DOSAGE: See prescribing information.
CAUTIONS: Check for minute leaks by squeezing bag firmly. If leaks are found, discard bag
as sterility may be impaired. Do not use unless solution is clear. Do not add
supplemental medication. Must not be used in series connections.
as sterility may be impaired. Do not use unless solution is clear. Do not add
supplemental medication. Must not be used in series connections.
STORAGE: Store at controlled room temperature 20º to 25º C (68º to 77º F); refer to USP
Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
U.S. Patent Numbers 7612102 and 7659291
Manufactured and Marketed by:
To report suspected adverse reactions, contact Baxter Healthcare Corporation
07-01-00-1497
CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
40 mg in 200 mL
CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
40 mg in 200 mL
DOUBLE CONCENTRATION:
CARDENE I.V.Nicardipine Hydrochloride
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
40 mg in 200 mL