Brand Name
Xenleta
Generic Name
Lefamulin Acetate
View Brand Information FDA approval date: September 09, 2019
Classification: Pleuromutilin Antibacterial
Form: Injection, Tablet
What is Xenleta (Lefamulin Acetate)?
XENLETA is a pleuromutilin antibacterial indicated for the treatment of adults with community-acquired bacterial pneumonia caused by susceptible microorganisms.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Xenleta (lefamulin acetate)
1DOSAGE FORMS AND STRENGTHS
XENLETA Injection
Clear, colorless solution in a single-dose clear glass vial. Each vial contains 150 mg of lefamulin in 15 mL of 0.9% sodium chloride for further dilution
XENLETA Tablets
Blue, oval, film-coated tablet with ‘LEF 600’ printed in black on one side. Each tablet contains 600 mg of lefamulin.
2ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QT Prolongation
- Clostridioides difficile-associated Diarrhea [see Warnings and Precautions (.
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
XENLETA was evaluated in two clinical trials in CABP patients (Trial 1 and Trial 2). Across the two trials, a total of 641 patients were treated with XENLETA. Trial 1 (intravenous [IV] to oral dosing switch trial) enrolled 551 adult patients, 276 randomized to XENLETA (273 received at least one dose of XENLETA) and 275 randomized to moxifloxacin (273 received at least one dose of moxifloxacin). Trial 2 (oral dosing only trial) enrolled 738 adult patients, 370 randomized to XENLETA (368 received at least one dose of XENLETA) and 368 randomized to moxifloxacin (all 368 received at least one dose of moxifloxacin).
Trial 1 enrolled patients with Pneumonia Outcomes Research Team (PORT) Risk Class III-V. The mean duration of intravenous treatment was 6 days; the mean total duration of treatment was 7 days. Trial 2 enrolled patients with PORT Risk Class II-IV. The mean duration of treatment was 5 days for XENLETA and 7 days for moxifloxacin.
In Trial 1 and Trial 2 (pooled), the median age of patients treated with XENLETA was 61 (range 19-97) years; 42% of patients were 65 years or older and 18% were 75 years or older. Patients were predominantly male (58%) and white (79%) and had a median body mass index (BMI) of 26.0 (range 13.0-56.8) kg/m
Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation
In Trial 1 and Trial 2 (pooled), serious adverse reactions occurred in 36/641 (5.6%) patients treated with XENLETA and 31/641 (4.8%) patients treated with moxifloxacin. Treatment was discontinued due to an adverse reaction in 21/641 (3.3%) patients treated with XENLETA and 21/641 (3.3%) patients treated with moxifloxacin. Death within 28 days occurred in 8/641 (1.2%) patients treated with XENLETA and 7/641 (1.1%) patients treated with moxifloxacin.
Most Common Adverse Reactions
Table 2 and Table 3 include adverse reactions occurring in ≥2% of patients receiving XENLETA in Trials 1 and 2.
Selected Adverse Reactions Occurring in Less Than 2% of Patients Receiving XENLETA in Trials 1 and 2
Blood and Lymphatic System Disorders: anemia, thrombocytopenia
Cardiac Disorders: atrial fibrillation, palpitations
Gastrointestinal Disorders: abdominal pain, constipation, dyspepsia, epigastric discomfort, erosive gastritis
Infections and Infestations: Clostridioides difficile colitis, oropharyngeal candidiasis, vulvovaginal candidiasis
Investigations: alkaline phosphatase increased, creatine phosphokinase increased, electrocardiogram QT prolonged, gamma-glutamyl transferase increased
Nervous System Disorders: somnolence
Psychiatric Disorders: anxiety
Renal and Urinary Disorders: urinary retention
3OVERDOSAGE
Treatment of overdose with XENLETA should consist of observation and general support measures. Lefamulin and its primary metabolite are not dialyzable.
4DESCRIPTION
XENLETA is a semi-synthetic antibacterial agent for oral and intravenous administration.
XENLETA, a pleuromutilin derivative, is available as 14-
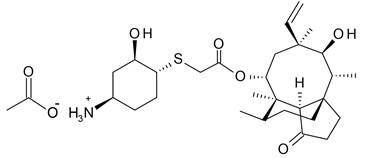
XENLETA Tablets for oral administration are available as blue, oval, film-coated tablets containing 671 mg lefamulin acetate equivalent to 600 mg lefamulin. The inactive ingredients are colloidal silicon dioxide, croscarmellose sodium, FD&C Blue No 2 aluminum lake, ferrosoferric oxide, magnesium stearate, mannitol, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol (partially hydrolyzed), povidone K30, shellac glaze, talc, and titanium dioxide.
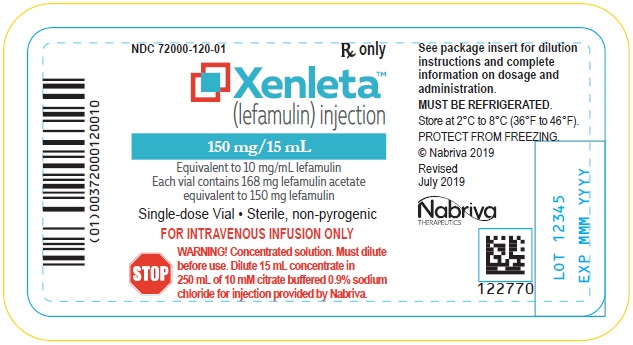
XENLETA Injection supplied as a sterile injection for intravenous use is available as a clear colorless solution in a glass vial containing 168 mg of lefamulin acetate equivalent to 150 mg of lefamulin in 15 mL of 0.9% sodium chloride. This is equivalent to 10 mg/mL lefamulin. The inactive ingredients are sodium chloride and water for injection.
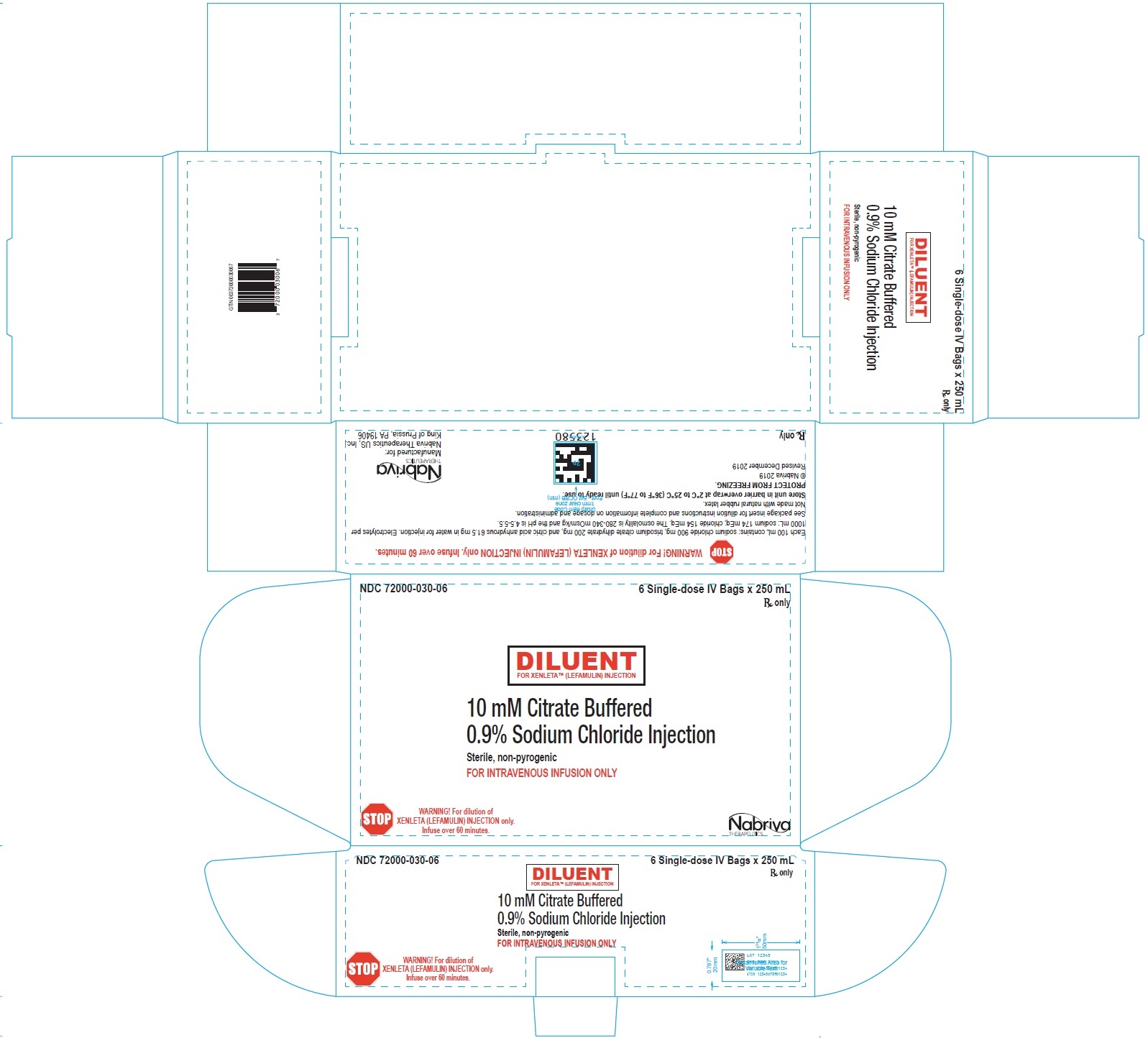
XENLETA Injection must be diluted with the diluent supplied with XENLETA Injection, before administration by intravenous infusion. Each supplied diluent infusion bag contains 250 mL of 10 mM citrate buffered (pH 5) 0.9% sodium chloride. The diluent is a clear, colorless solution. The inactive ingredients are citric acid anhydrous, sodium chloride, trisodium citrate dihydrate, and water for injection. Each 100 mL contains: sodium chloride 900 mg, trisodium citrate dihydrate 200 mg, and citric acid anhydrous 61.5 mg in water for injection. Electrolytes per 1000 mL: sodium 174 mEq; chloride 154 mEq. The osmolality is 280-340 mOsm/kg and the pH is 4.5-5.5.
5HOW SUPPLIED/STORAGE AND HANDLING
XENLETA is supplied in the following strengths and package configurations:
XENLETA Injection
How Supplied
XENLETA Injection is a clear, colorless, sterile, nonpyrogenic solution for intravenous administration containing 150 mg of lefamulin in 15 mL 0.9% sodium chloride in a single-dose vial intended for dilution in 250 mL of 10 mM citrate buffered (pH 5) 0.9% sodium chloride. The drug product is provided in a clear type I glass 15 mL vial with a gray rubber stopper, aluminum seal and flip off cap. The diluent is provided in infusion bags containing 250 mL of sterile, nonpyrogenic 10 mM citrate buffered (pH 5) 0.9% sodium chloride solution. The vial stopper and infusion bag are not made with natural rubber latex.
They are supplied as follows:
150 mg single dose lefamulin vials (NDC 72000-120-06); packed in cartons of 6.
250 mL citrate buffer diluent bags (NDC 72000-030-06); packed in cartons of 6.
Storage and Handling
XENLETA Injection should be stored at 2°C to 8°C (36°F to 46°F). Store in a refrigerator. Do not freeze. The diluent bags should be stored in barrier overwrap at 2°C to 25°C (36°F to 77°F) until ready to use.
XENLETA Tablets
How Supplied
XENLETA Tablets are available as blue, oval, film-coated tablets containing 600 mg lefamulin. The tablets are printed with ‘LEF 600’ in black on one side.
They are supplied as follows:
Child-resistant blister cards of 10 tablets (NDC 72000-110-10).
HDPE bottles of 30 tablets with Child-resistant Closure (NDC 72000-110-30).
Storage and Handling
XENLETA Tablets should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
6PATIENT COUNSELING INFORMATION
Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterial drugs, including XENLETA, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with an antibacterial drug, patients can develop watery stools (with or without stomach cramps and fever) which may be a sign of a more serious intestinal infection, even as late as 2 or more months after having taken the last dose of the antibacterial drug. If this occurs, instruct patients to contact their healthcare provider as soon as possible
Nausea and Vomiting
Advise patients that nausea and vomiting are common adverse reactions to XENLETA
Drug Interactions
Advise patients of the potential interaction other medications can have with XENLETA or the effect XENLETA may have on other medications, as these interactions may result in decreased effectiveness or increased toxicities of either XENLETA or the other medications. Patients should alert their physician if they are currently taking any medication(s) (including herbal or nutritional supplements) or are prescribed new medication(s) during treatment with XENLETA
Allergic Reactions
Advise patients that allergic reactions, including serious allergic reactions, could occur with XENLETA and that serious allergic reactions require immediate treatment. Ask the patient about any previous hypersensitivity reactions to XENLETA, or other pleuromutilin class antibacterial drugs
Administration with Food
Advise patients that XENLETA should be taken at least 1 hour before a meal or 2 hours after a meal and should be swallowed whole with water (6 to 8 ounces). XENLETA should not be crushed or divided
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus, and to inform their healthcare provider of a known or suspected pregnancy. Advise patients to avoid becoming pregnant while receiving this drug
Advise females of reproductive potential to use effective contraception during treatment with XENLETA and for 2 days after the final dose
Inform patients that Nabriva Therapeutics has a surveillance program for pregnant women who have inadvertently taken XENLETA during pregnancy. Advise patients to call 1-855-5NABRIVA to enroll
Lactation
Advise lactating women to pump and discard human milk for the duration of treatment with XENLETA and for 2 days after the final dose
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including XENLETA should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When XENLETA is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of treatment, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by XENLETA or other antibacterial drugs in the future
Distributed by:
Nabriva Therapeutics US, Inc.
Fort Washington, PA 19034
Nabriva Therapeutics is a trademark of Nabriva Therapeutics US, Inc.
XENLETA is a trademark of Nabriva Therapeutics US, Inc.
For patent information:
Copyright © 2019-2021 Nabriva Therapeutics US, Inc., an affiliate of Nabriva Therapeutics Ireland DAC. All rights reserved.
7PRINCIPAL DISPLAY PANEL - NDC: 72000-120-01 - Injection Vial Label
8PRINCIPAL DISPLAY PANEL - NDC: 72000-120-06 - Injection Carton Label

9PRINCIPAL DISPLAY PANEL - NDC: 72000-030-01 - Diluent Bag Label

10PRINCIPAL DISPLAY PANEL - NDC: 72000-030-06 - Diluent Carton Label
11PRINCIPAL DISPLAY PANEL - NDC: 72000-110-30 - Tablet 30-count Bottle Label

12PRINCIPAL DISPLAY PANEL - NDC: 72000-110-10 - 10 Count Blister Pack Label
