Brand Name
Cinqair
Generic Name
Reslizumab
View Brand Information FDA approval date: April 18, 2016
Classification: Interleukin-5 Antagonist
Form: Injection
What is Cinqair (Reslizumab)?
CINQAIR ® is indicated for the add-on maintenance treatment of patients with severe asthma aged 18 years and older with an eosinophilic phenotype. Limitation of Use: CINQAIR is not indicated for treatment of other eosinophilic conditions. CINQAIR is not indicated for the relief of acute bronchospasm or status asthmaticus [see Warnings and Precautions.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Pilot Phase 2 Study of the Safety and Efficacy of Dupilumab as Add-on Therapy for Hypereosinophilic Syndrome With Partial Clinical Response to Eosinophil-Depleting Biologic Agents
Background: Hypereosinophilic syndrome (HES) is a blood disorder that causes high levels of white blood cells called eosinophils. HES can damage the lungs and airways, intestines, skin, and other organs. The current primary treatment for HES can cause serious side effects. Secondary treatments do not work in all people.
Related Latest Advances
Brand Information
CINQAIR (Reslizumab)
WARNING: ANAPHYLAXIS
Anaphylaxis has been observed with CINQAIR infusion in 0.3% of patients in placebo-controlled clinical studies. Anaphylaxis was reported as early as the second dose of CINQAIR
Anaphylaxis can be life-threatening. Patients should be observed for an appropriate period of time after CINQAIR administration by a healthcare professional prepared to manage anaphylaxis. Discontinue CINQAIR immediately if the patient experiences signs or symptoms of anaphylaxis
1INDICATIONS AND USAGE
CINQAIR
Limitation of Use:
- CINQAIR is not indicated for treatment of other eosinophilic conditions.
- CINQAIR is not indicated for the relief of acute bronchospasm or status asthmaticus
2DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/10 mL (10 mg/mL), clear to slightly hazy/opalescent, colorless to slightly yellow solution in single-use vials.
3CONTRAINDICATIONS
CINQAIR is contraindicated in patients who have known hypersensitivity to reslizumab or any of its excipients
4ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Anaphylaxis
- Malignancy
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Overall, 2195 subjects received at least 1 dose of CINQAIR. The data described below reflect exposure to CINQAIR in 1611 patients with asthma, including 1120 exposed for up to 16 weeks, 1006 exposed for 6 months, 759 exposed for 1 year, and 249 exposed for longer than 2 years. The above referenced safety exposure for CINQAIR is derived from placebo-controlled studies ranging from 15 to 52 weeks in duration (CINQAIR 0.3 mg/kg and 3 mg/kg [n=1131] and placebo [n=730]) and 480 new CINQAIR 3 mg/kg exposures (previously on placebo) from a single open-label extension study (n=1051). While a lower dose of CINQAIR 0.3 mg/kg (n=103) was included in a clinical trial, 3 mg/kg is the only recommended dose
Serious adverse reactions that occurred in placebo-controlled studies in more than 1 subject and in a greater percentage of subjects treated with CINQAIR (n=1131) than placebo (n=730) included anaphylaxis (3 subjects vs. 0 subjects, respectively). The 3 subjects who experienced anaphylaxis were discontinued from the clinical studies
Adverse reactions that occurred at greater than or equal to 2% incidence and more commonly than in the placebo group included 1 event: oropharyngeal pain (2.6% vs. 2.2%).
CPK elevations and muscle-related adverse reactions
Elevated baseline creatine phosphokinase (CPK) was more frequent in patients randomized to CINQAIR (14%) versus placebo (9%). Transient CPK elevations in patients with normal baseline CPK values were observed more frequently with CINQAIR (20%) versus placebo (18%) during routine laboratory assessments. CPK elevations >10 x ULN, regardless of baseline CPK value, were 0.8% in the CINQAIR group compared to 0.4% in the placebo group. CPK elevations >10 x ULN were asymptomatic and did not lead to treatment discontinuation.
Myalgia was reported in 1% (10/1028) of patients in the CINQAIR 3 mg/kg group compared to 0.5% (4/730) of patients in the placebo group. On the day of infusion, musculoskeletal adverse reactions were reported in 2.2% and 1.5% of patients treated with CINQAIR 3 mg/kg and placebo, respectively. These reactions included (but were not limited to) musculoskeletal chest pain, neck pain, muscle spasms, extremity pain, muscle fatigue, and musculoskeletal pain.
4.2Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. In placebo-controlled studies, a treatment-emergent anti-reslizumab antibody response developed in 53/983 (5.4%) of CINQAIR-treated patients (3 mg/kg). In the long-term, open-label study, treatment-emergent anti-reslizumab antibodies were detected in 49/1014 (4.8%) of CINQAIR-treated (3 mg/kg) asthma patients over 36 months. The antibody responses were of low titer and often transient. Neutralizing antibodies were not evaluated. There was no detectable impact of the antibodies on the clinical pharmacokinetics, pharmacodynamics, clinical efficacy, and safety of CINQAIR
The data reflect the percentage of patients whose test results were positive for antibodies to reslizumab in specific assays. The observed incidence of antibody response is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to reslizumab with the incidence of antibodies to other products may be misleading.
5DRUG INTERACTIONS
No formal clinical drug interaction studies have been performed with CINQAIR.
6OVERDOSAGE
Single doses of up to 732 mg have been administered intravenously to subjects in clinical trials without evidence of dose-related toxicities.
There is no specific treatment for an overdose with CINQAIR. If overdose occurs, the patient should be treated supportively with appropriate monitoring as necessary.
7DESCRIPTION
CINQAIR (reslizumab) is a humanized interleukin-5 antagonist monoclonal antibody (IgG4κ). Reslizumab is produced by recombinant DNA technology in murine myeloma non-secreting 0 (NS0) cells. Reslizumab has a molecular weight of approximately 147 kDa.
CINQAIR is a sterile, preservative-free, clear to slightly hazy/opalescent, colorless to slightly yellow solution (injection) for intravenous infusion. Since CINQAIR is a protein, proteinaceous particles may be present in the solution that appear as translucent to white, amorphous particulates. Each single-use vial contains 100 mg reslizumab in 10 mL. Each mL contains 10 mg of reslizumab, glacial acetic acid (0.12 mg), sodium acetate trihydrate (2.45 mg), and sucrose (70 mg), with a pH of 5.5.
8CLINICAL STUDIES
The asthma development program for CINQAIR 3 mg/kg (administered once every 4 weeks) included 4 randomized, double-blind, placebo-controlled studies (Studies I-IV) 16 to 52 weeks in duration involving 981 patients 12 years of age and older. While patients aged 12 to 17 years were included in these trials, CINQAIR is not approved for use in this age group
Studies I and II
Studies I and II were 52-week studies in 953 patients with asthma who were required to have a blood eosinophil count of at least 400/mcL (within 3 to 4 weeks of dosing), and at least 1 asthma exacerbation requiring systemic corticosteroid use over the past 12 months. The majority of patients (82%) were on medium-high dose inhaled corticosteroids plus a long-acting beta agonist (ICS/LABA) at baseline. Maintenance oral corticosteroids (OCS) (up to 10 mg of prednisone per day or equivalent) were allowed; 106 (11%) patients were on OCS at baseline. CINQAIR 3 mg/kg administered once every 4 weeks for a total of 13 doses was evaluated compared with placebo.
Study III
Study III was a 16-week study in 315 patients who were required to have a blood eosinophil count of at least 400/mcL at screening (within 3 to 4 weeks of dosing). Maintenance OCS were not allowed. CINQAIR 3 mg/kg or 0.3 mg/kg administered once every 4 weeks for a total of 4 doses was evaluated compared with placebo. While 2 doses of CINQAIR were studied, CINQAIR 3 mg/kg is the only recommended dose
Study IV
Study IV was a 16-week study in 496 patients unselected for baseline blood eosinophil levels (approximately 80% of patients had a screening [within 3 to 4 weeks of dosing] blood eosinophil count of less than 400/mcL). Maintenance OCS were not allowed. CINQAIR 3 mg/kg administered once every 4 weeks for a total of 4 doses was evaluated compared with placebo.
The demographics and baseline characteristics of these 4 studies is provided in Table 1.
Table 1: Demographics and Baseline Characteristics of Patients in Asthma Studies
FEV
All patients had to be on inhaled corticosteroid (ICS) background therapy and could have been receiving any combination of background therapies (ICS with or without another controller [non-ICS and/or OCS]).
Exacerbations
The primary endpoint for Studies I and II was the frequency of asthma exacerbations for each patient during the 52-week treatment period. An asthma exacerbation was defined as a worsening of asthma that required at least 1 of the following medical interventions:
1) Either the use of a systemic corticosteroid, or ≥ 2-fold an increase in the use of ICS for 3 or more days, and/or
2) Asthma-related emergency treatment including at least 1 of the following: an unscheduled visit to their healthcare professional for nebulizer treatment or other urgent treatment to prevent worsening of asthma symptoms; a visit to the emergency room for asthma-related treatment; or an asthma-related hospitalization.
The medical intervention had to be corroborated with at least 1 of the following: 1) a decrease in forced expiratory volume in 1 second (FEV
In Studies I and II, patients receiving CINQAIR 3 mg/kg administered once every 4 weeks had significant reductions in the rate of all asthma exacerbations compared to placebo (Table 2). Exacerbations requiring the use of a systemic corticosteroid (e.g., OCS) as well as exacerbations resulting in hospitalization or an emergency room visit were each reduced with CINQAIR 3 mg/kg.
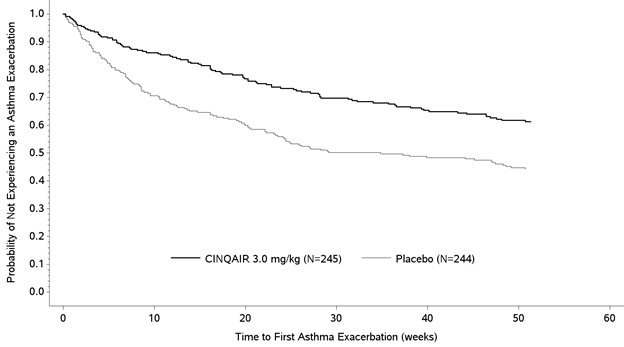
The proportion of patients who did not experience an asthma exacerbation during the 52-week treatment period was higher in the CINQAIR 3 mg/kg group (62% and 75%) compared with the placebo group (46% and 55%), in Studies I and II, respectively. The time to first asthma exacerbation was significantly longer for the groups receiving CINQAIR 3 mg/kg compared with placebo in both Studies I and II. A representative figure from Study I is shown below (Figure 1). Study II showed similar results.
Figure 1: Time to First Asthma Exacerbation by Treatment Group in Patients with Severe Asthma with an Eosinophilic Phenotype (Study I)
Lung Function
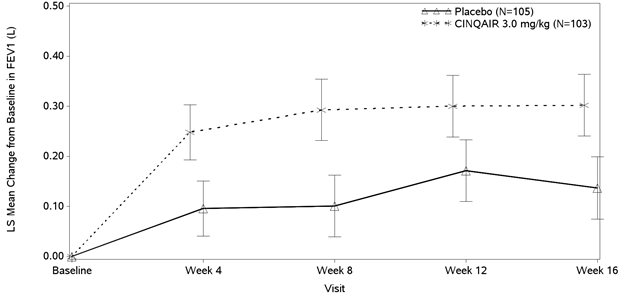
The effect of CINQAIR 3 mg/kg administered once every 4 weeks on FEV
Study III also studied a lower dose, CINQAIR 0.3 mg/kg, that produced significant but numerically smaller changes in FEV
Study IV was the only study to test CINQAIR 3 mg/kg in asthma patients unselected for blood eosinophils (measured 3 to 4 weeks prior to dosing); association of treatment effect (i.e., difference between CINQAIR and placebo in the change in FEV
Table 3: Mean Change (95% CI) from Baseline in FEV
Improvements in FEV
Figure 2: Mean Change from Baseline in FEV
The Asthma Control Questionnaire-7 (ACQ-7) and Asthma Quality of Life Questionnaire (AQLQ) were both assessed in Studies I, II, and III. The responder rate for both measures was defined as an improvement in score of 0.5 or more as threshold over 16 weeks.
- For ACQ-7, the responder rate for those randomized to CINQAIR vs. placebo was 69% vs. 65% for Study I, 70% vs. 58% for Study II, and 64% vs. 58% for Study III.
- For AQLQ, the responder rate for those randomized to CINQAIR vs. placebo was 66% vs. 58% for Study I, 67% vs. 55% for Study II, and 64% vs. 48% for Study III.
9HOW SUPPLIED/STORAGE AND HANDLING
CINQAIR (reslizumab) injection, 100 mg/10 mL (10 mg/mL), is supplied as a preservative-free, sterile, clear to slightly hazy/opalescent, colorless to slightly yellow solution in single-use vials. The following packaging configuration is available:
- NDC 59310-610-31: 100 mg/10 mL (10 mg/mL) single-use vial.
Storage and Stability: Refrigerate at 2 ºC to 8ºC (36°F to 46°F). Do not freeze. Do not shake. Protect the vials from light by storing in the original package until time of use.
10PATIENT COUNSELING INFORMATION
See FDA approved patient labeling (Patient Information).
Hypersensitivity/Anaphylaxis
Inform patients that hypersensitivity reactions, including anaphylaxis, have occurred with administration of CINQAIR. Educate patients on the signs and symptoms of hypersensitivity reactions and anaphylaxis (e.g., skin or mucosal involvement, airway compromise, reduced blood pressure). Instruct patients to contact their healthcare professional immediately if they experience symptoms of an allergic reaction after they have received their infusion of CINQAIR
Not for Acute Symptoms or Deteriorating Disease
Inform patients that CINQAIR does not treat acute asthma symptoms or acute exacerbations. Inform patients to seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with CINQAIR
Malignancy
Counsel CINQAIR-treated patients about the risk of malignancies
Reduction of Corticosteroid Dosage
Inform patients not to discontinue systemic or inhaled corticosteroids except under the direct supervision of a physician. Inform patients that reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy
Manufactured by:
Teva Respiratory, LLC
West Chester, PA 19380
U.S. License Number 2047
US Patent RE39,548 and 6,056,957
©2020 Teva Respiratory, LLC
CNQ-004
11Patient Package Insert
PATIENT INFORMATION
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 02/2020
12Package/Label Display Panel NDC 59310-610-31
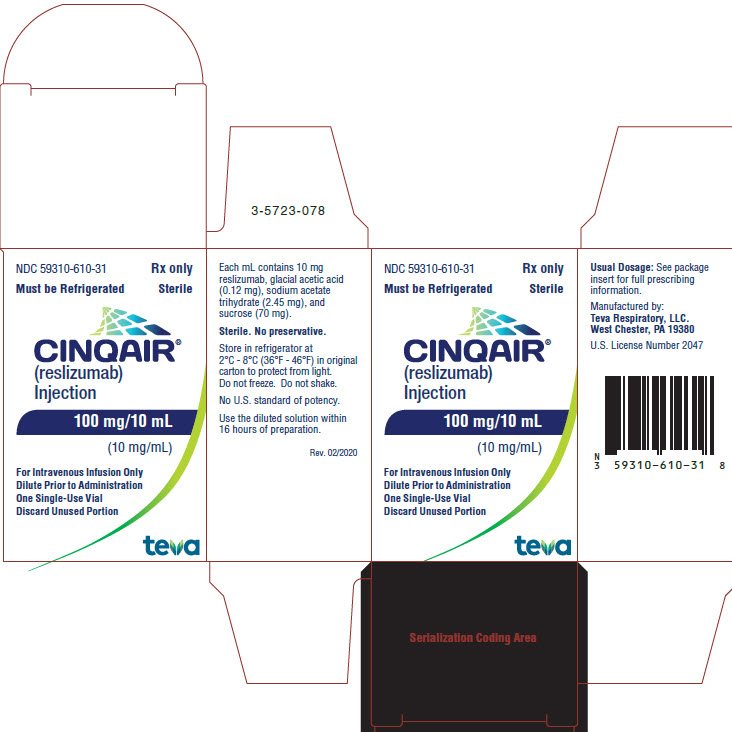
NDC 59310-610-31
Rx only
Must be Refrigerated Sterile
CINQAIR® (reslizumab) Injection 100 mg/10 mL (10 mg/mL)
For Intravenous Infusion Only
Dilute Prior to Administration
One Single-Use Vial
Discard Unused Portion
TEVA
13Package/Label Display Panel NDC 59310-610-33
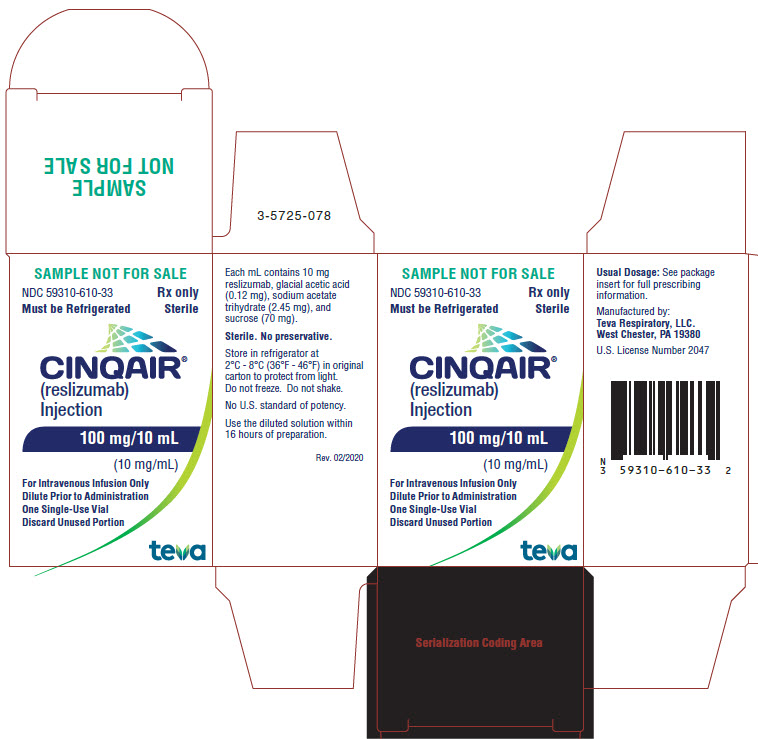
NDC 59310-610-33
Rx only
Must be Refrigerated
Sterile
CINQAIR® (reslizumab) Injection 100 mg/10 mL (10 mg/mL)
For Intravenous Infusion Only
Dilute Prior to Administration
One Single-Use Vial
Discard Unused Portion
TEVA



