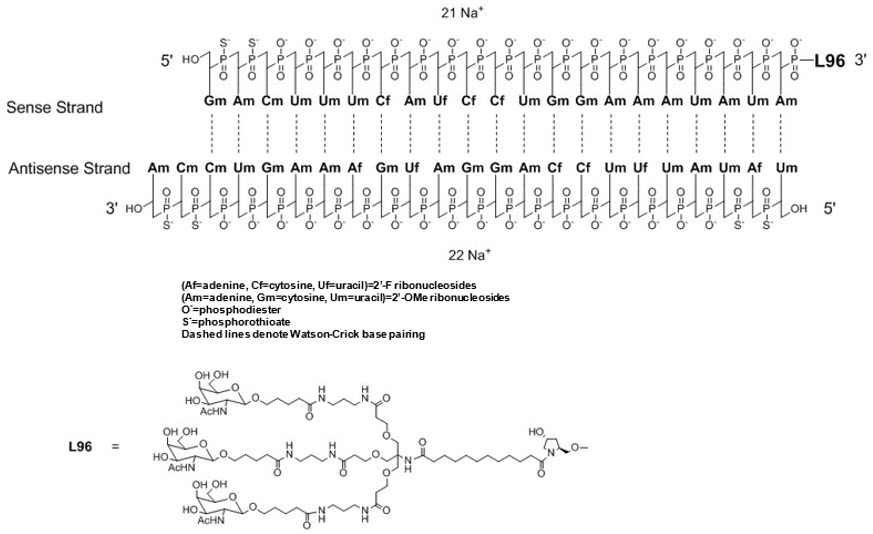
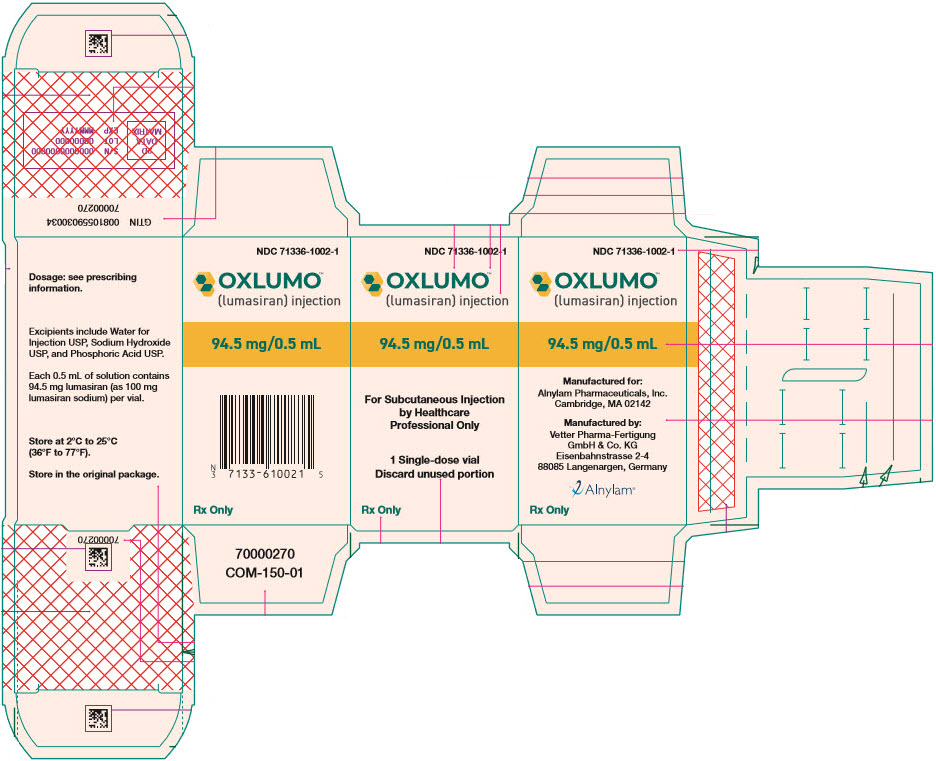
Oxlumo
What is Oxlumo (Lumasiran)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of this study is to describe the natural history and progression of patients diagnosed with PH1, and to characterize the long-term real-world safety and effectiveness of lumasiran.
Summary: This study will look at how well a drug that reduced the amount of oxalate in the body works in patients that have kidney disease and need dialysis treatment. People with kidney disease often have higher levels of oxalate in the blood. People with kidney disease are also at higher risk of having heart attacks, heart disease and strokes (these are called cardiovascular diseases). It is thought that...
Summary: Primary hyperoxaluria type 1 (PH1) is a rare genetic disease caused by mutation in the AGXT gene encoding the hepatic peroxisomal enzyme AGT. Reduced AGT activity results in increased glyoxylate and oxalate production, causing the formation of kidney stones, nephrocalcinosis and renal failure. Clinical trials of Lumasiran have provided information on the efficacy and safety of Lumasiran in the tre...
Related Latest Advances
Brand Information