Brand Name
Fareston
Generic Name
Toremifene
View Brand Information FDA approval date: June 30, 1997
Classification: Estrogen Agonist/Antagonist
Form: Tablet
What is Fareston (Toremifene)?
FARESTON® is an estrogen agonist/antagonist indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors. FARESTON® is an estrogen agonist/antagonist indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Fareston (toremifene citrate)
WARNING: QT PROLONGATION
FARESTON has been shown to prolong the QTc interval in a dose- and concentration-related manner
1INDICATIONS AND USAGE
FARESTON® is an estrogen agonist/antagonist indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors.
2DOSAGE AND ADMINISTRATION
The dosage of FARESTON is 60 mg, once daily, orally. Treatment is generally continued until disease progression is observed.
3DOSAGE FORMS AND STRENGTHS
Tablet is 60 mg, round, convex, unscored, uncoated, and white, or almost white, identified with TO 60 embossed on one side.
4ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
4.1Clinical Trials Experience
Adverse drug reactions are principally due to the antiestrogenic actions of FARESTON and typically occur at the beginning of treatment.
The incidences of the following eight clinical toxicities were prospectively assessed in the North American Study. The incidence reflects the toxicities that were considered by the investigator to be drug related or possibly drug related.
Approximately 1% of patients receiving FARESTON (n = 592) in the three controlled studies discontinued treatment as a result of adverse reactions (nausea and vomiting, fatigue, thrombophlebitis, depression, lethargy, anorexia, ischemic attack, arthritis, pulmonary embolism, and myocardial infarction).
Serious adverse reactions occurring in at least 1% of patients receiving FARESTON in the three major trials are listed in the table below.
Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted. The patients were randomized to parallel groups receiving FARESTON 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively
Other adverse reactions included leukopenia and thrombocytopenia, skin discoloration or dermatitis, constipation, dyspnea, paresis, tremor, vertigo, pruritus, anorexia, reversible corneal opacity (corneal verticulata), asthenia, alopecia, depression, jaundice, and rigors.
The incidence of AST elevations was greater in the 200 and 240 mg FARESTON dose arms than in the tamoxifen arms. Higher doses of FARESTON were also associated with an increase in nausea.
Approximately 4% of patients were withdrawn for toxicity from the high-dose FARESTON treatment arms. Reasons for withdrawal included hypercalcemia, abnormal liver function tests, and one case each of toxic hepatitis, depression, dizziness, incoordination, ataxia, blurry vision, diffuse dermatitis, and a constellation of symptoms consisting of nausea, sweating, and tremor.
4.2Post-marketing Experience
The following adverse reactions were identified during post approval use of FARESTON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported during post approval use of FARESTON have been consistent with clinical trial experience. The most frequently reported adverse reactions related to FARESTON use since market introduction include hot flash, sweating, nausea, and vaginal discharge.
Hepatotoxicity
Risk of Uterine Malignancy
Hypertriglyceridemia
5OVERDOSAGE
Lethality was observed in rats following single oral doses that were ≥1000 mg/kg (about 150 times the recommended human dose on a mg/m
Vertigo, headache, and dizziness were observed in healthy volunteer studies at a daily dose of 680 mg for 5 days. The symptoms occurred in two of the five subjects during the third day of the treatment and disappeared within 2 days of discontinuation of the drug. No immediate concomitant changes in any measured clinical chemistry parameters were found. In a study in postmenopausal breast cancer patients, toremifene 400 mg/m
Theoretically, overdose may be manifested as an increase of antiestrogenic effects, such as hot flashes; estrogenic effects, such as vaginal bleeding; or nervous system disorders, such as vertigo, dizziness, ataxia, and nausea. There is no specific antidote and the treatment is symptomatic.
6DESCRIPTION
FARESTON (toremifene citrate) Tablets for oral administration each contain 88.5 mg of toremifene citrate, which is equivalent to 60 mg toremifene.
FARESTON is an estrogen agonist/antagonist. The chemical name of toremifene is: 2-{p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy}-N,N-dimethylethylamine citrate (1:1). The structural formula is:
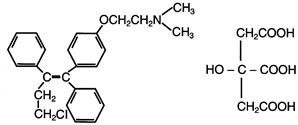
and the molecular formula is C
FARESTON is available only as tablets for oral administration. Inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate, and starch.
7CLINICAL STUDIES
Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted to evaluate the efficacy of FARESTON for the treatment of breast cancer in postmenopausal women. The patients were randomized to parallel groups receiving FARESTON 60 mg (FAR60) or tamoxifen 20 mg (TAM20) in the North American Study or tamoxifen 40 mg (TAM40) in the Eastern European and Nordic studies. The North American and Eastern European studies also included high-dose toremifene arms of 200 and 240 mg daily, respectively. The studies included postmenopausal patients with estrogen-receptor (ER) positive or estrogen-receptor (ER) unknown metastatic breast cancer. The patients had at least one measurable or evaluable lesion. The primary efficacy variables were response rate (RR) and time to progression (TTP). Survival (S) was also determined. Ninety-five percent confidence intervals (95% CI) were calculated for the difference in RR between FAR60 and TAM groups and the hazard ratio (relative risk for an unfavorable event, such as disease progression or death) between TAM and FAR60 for TTP and S.
Two of the 3 studies showed similar results for all effectiveness endpoints. However, the Nordic Study showed a longer time to progression for tamoxifen (see table).
The high-dose groups, toremifene 200 mg daily in the North American Study and 240 mg daily in the Eastern European Study, were not superior to the lower toremifene dose groups, with response rates of 22.6% and 28.7%, median times to progression of 5.6 and 6.1 months, and median survivals of 30.1 and 23.8 months, respectively. The median treatment duration in the three pivotal studies was 5 months (range 4.2-6.3 months).
8SUPPLIED/STORAGE AND HANDLING
FARESTON Tablets, containing toremifene citrate in an amount equivalent to 60 mg of toremifene, are round, convex, unscored, uncoated, and white, or almost white.
FARESTON Tablets are identified with TO 60 embossed on one side.
FARESTON Tablets are available as:
Store at 25°C (77°F).
Excursions permitted to 15-30°C (59-86°F)
[See USP Controlled Room Temperature.]
Protect from heat and light.
Excursions permitted to 15-30°C (59-86°F)
[See USP Controlled Room Temperature.]
Protect from heat and light.
9PATIENT COUNSELING INFORMATION
Vaginal bleeding has been reported in patients using FARESTON. Patients should be informed about this and instructed to contact their physician if such bleeding or other gynecological symptoms (changes in vaginal discharge, pelvic pain or pressure) occur. Patients should have a gynecological examination prior to initiation of therapy and at regular intervals while on therapy.
Liver disorders including transaminits grade 3 and 4, hyperbilirubinemia with jaundice have been reported in patients using FARESTON. Patients should have liver function tests performed periodically while on therapy.
FARESTON may harm the fetus and increase the risk for pregnancy loss
Premenopausal women using FARESTON should use nonhormonal contraception during treatment and should be apprised of the potential hazard to the fetus should pregnancy occur
Patients with bone metastases should be informed about the typical signs and symptoms of hypercalcemia and instructed to contact their physician for further assessment if such signs or symptoms occur.
Patients who must take medications known to prolong the QT interval, or potent CYP3A4 inhibitors, should be informed of the effect of toremifene on QT interval. Toremifene has been shown to prolong the QTc interval in a dose-related manner
Specific interactions with foods that inhibit CYP3A4, including grapefruit juice, have not been studied but may increase toremifene concentrations. Patients should avoid grapefruit products and other foods that are known to inhibit CYP3A4 during FARESTON treatment.
Certain other medicines, including over-the-counter medications or herbal supplements (such as St. John's Wort) and toremifene, can reduce concentrations of co-administered drugs
10PRINCIPAL DISPLAY PANEL - 60 mg Tablet Carton
NDC 42747-327-30
Fareston
30 TABLETS
Rx only
60 mg
KYOWA KIRIN