Brand Name
Daurismo
Generic Name
Glasdegib
View Brand Information FDA approval date: December 10, 2018
Classification: Hedgehog Pathway Inhibitor
Form: Tablet
What is Daurismo (Glasdegib)?
DAURISMO is indicated, in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid leukemia in adult patients who are ≥75 years old or who have comorbidities that preclude use of intensive induction chemotherapy. DAURISMO is a hedgehog pathway inhibitor indicated, in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid leukemia in adult patients who are ≥75 years old or who have comorbidities that preclude use of intensive induction chemotherapy.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Phase 3 Study to Assess Gemtuzumab, in Combination With Standard Chemotherapy, on MRD Levels, in Adult, 18-60 Years, With Previously Untreated de Novo Fav-interm Risk AML
Summary: MRD driven study. Addition of gemtuzumab to conventional chemotherapy to reduce MRD of patients with favorable/intermediate-risk AML. Post-consolidation assessment of MRD.
Related Latest Advances
Brand Information
Daurismo (glasdegib)
WARNING: EMBRYO-FETAL TOXICITY
DAURISMO can cause embryo-fetal death or severe birth defects when administered to a pregnant woman. DAURISMO is embryotoxic, fetotoxic, and teratogenic in animals
Conduct pregnancy testing in females of reproductive potential prior to initiation of DAURISMO treatment. Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and for at least 30 days after the last dose
Advise males of the potential risk of DAURISMO exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the last dose to avoid potential drug exposure
1INDICATIONS AND USAGE
DAURISMO is indicated, in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adult patients who are ≥75 years old or who have comorbidities that preclude use of intensive induction chemotherapy.
2DOSAGE FORMS AND STRENGTHS
DAURISMO 100 mg tablets: round, pale orange film-coated tablet debossed with "Pfizer" on one side and "GLS 100" on the other.
DAURISMO 25 mg tablets: round, yellow film-coated tablet debossed with "Pfizer" on one side and "GLS 25" on the other.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically-significant adverse reactions are described elsewhere in the labeling:
- QTc Interval Prolongation
- Musculoskeletal Adverse Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety profile of DAURISMO is based on experience in the BRIGHT AML 1003 study for 111 adults with newly-diagnosed AML and 14 adults with other conditions for which DAURISMO is not indicated
Serious adverse reactions were reported in 79% of patients treated in the DAURISMO with low-dose cytarabine arm. The most common (≥5%) serious adverse reactions in patients receiving DAURISMO with low-dose cytarabine were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%), and sepsis (7%).
Dose reductions associated with adverse reactions were reported in 26% of patients treated with DAURISMO with low-dose cytarabine, and the most common reasons (≥2%) for dose reductions due to adverse reactions were muscle spasms (5%), fatigue (4%), febrile neutropenia (4%), anemia (2%), thrombocytopenia (2%), and ECG QT prolonged (2%). Adverse reactions leading to permanent discontinuation were reported in 36% of patients treated with DAURISMO with low-dose cytarabine, and the most common (≥2%) reasons for permanent discontinuation were pneumonia (6%), febrile neutropenia (4%), sepsis (4%), sudden death (2%), myocardial infarction (2%), nausea (2%), and renal insufficiency (2%).
Adverse reactions reported in the first 90 days of therapy on the BRIGHT AML 1003 study are shown in Table 3.
The adverse reactions muscle spasms (4 in 12 patients) and decreased appetite (2 in 10 patients) worsened (i.e. progressed from Grades ≤2 to Grade 3 or higher) after the first 90 days of therapy in BRIGHT AML 1003.
Additional clinically-significant adverse reactions occurring in <10% of patients treated with DAURISMO and low-dose cytarabine in BRIGHT AML 1003 include:
- Dental disorders: loose tooth and toothache
- Skin and subcutaneous tissue disorders: alopecia
- Cardiac disorders: QT interval prolonged
Changes in selected post-baseline laboratory values that were observed in patients with newly-diagnosed AML and other conditions for which DAURISMO is not indicated in the clinical trial are shown in Table 4.
The following laboratory abnormalities worsened (i.e. progressed from Grades ≤2 to Grade 3 or higher) after the first 90 days of therapy in BRIGHT AML 1003:
- hypophosphatemia (8 in 38 patients), creatinine increased (2 in 39 patients), and ALT increased (2 in 40 patients).
5OVERDOSAGE
There is no specific antidote for DAURISMO. Management of DAURISMO overdose should include symptomatic treatment and ECG monitoring.
Glasdegib has been administered in clinical studies up to a dose of 640 mg/day. At the highest dosage, the adverse reactions that were dose limiting were nausea, vomiting, dehydration, hypotension, fatigue, and dizziness.
6DESCRIPTION
DAURISMO (glasdegib) is a hedgehog pathway inhibitor. It is formulated with the maleate salt of glasdegib. The molecular formula for glasdegib maleate is C
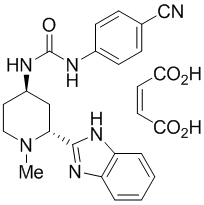
Glasdegib maleate is a white to pale colored powder with pKa values of 1.7 and 6.1. The aqueous solubility of glasdegib maleate is 1.7 mg/mL.
DAURISMO (glasdegib) is supplied as a film-coated tablet for oral use containing either 100 mg glasdegib (equivalent to 131.1 mg glasdegib maleate) or 25 mg of glasdegib (equivalent to 32.8 mg glasdegib maleate) together with microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and magnesium stearate as inactive ingredients in the tablet. The film-coating consists of Opadry II
7CLINICAL STUDIES
The efficacy of DAURISMO in combination with low-dose cytarabine was evaluated in a multicenter, open-label, randomized study (Study BRIGHT AML 1003, NCT01546038) that included 115 patients age 55 years or older with newly-diagnosed AML who met at least one of the following criteria: a) age ≥75 years, b) severe cardiac disease, c) baseline Eastern Cooperative Oncology Group (ECOG) performance status of 2, or d) baseline serum creatinine >1.3 mg/dL. Patients were randomized 2:1 to receive DAURISMO at a 100 mg daily dose with low-dose cytarabine 20 mg subcutaneously twice daily on days 1 to 10 of a 28-day cycle (N=77) or low-dose cytarabine alone (N=38) in 28-day cycles until disease progression or unacceptable toxicity. Patients were stratified by cytogenetic risk (good/intermediate or poor).
The baseline demographic and disease characteristics are shown in Table 6. The two treatment arms were generally balanced with respect to the baseline demographics and disease characteristics (see Table 6).
Efficacy was established on the basis of overall survival (OS) from the date of randomization to death from any cause. With a median follow-up of approximately 20 months, the DAURISMO with low-dose cytarabine arm was superior to low-dose cytarabine alone arm (Figure 1). The efficacy results are shown in Table 7. Improvement in OS was consistent across prespecified cytogenetic risk subgroups.
Figure 1. BRIGHT AML 1003 – Kaplan-Meier Plot of Overall Survival for Patients with AML
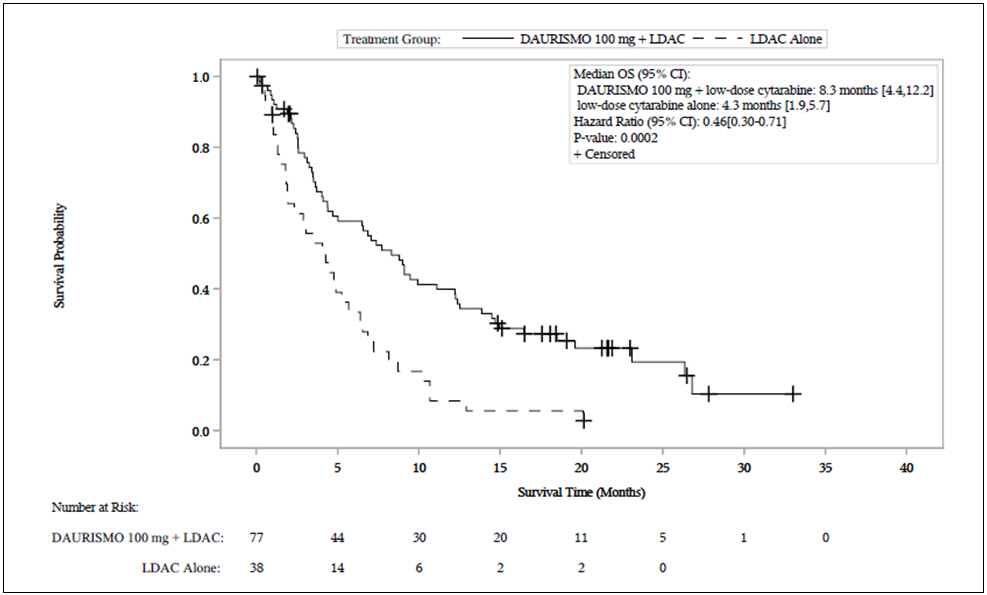
Abbreviations: CI = confidence interval; OS = overall survival; LDAC = low-dose cytarabine.
8HOW SUPPLIED/STORAGE AND HANDLING
DAURISMO is supplied in the following strengths and package configurations:
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
10PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label
ALWAYS DISPENSE
NDC 0069-0298-60
Pfizer
Daurismo™
25 mg*
Do not cut, crush, or chew the tablets.
60 Tablets

11PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
ALWAYS DISPENSE
NDC 0069-1531-30
Pfizer
Daurismo™
100 mg*
Do not cut, crush, or chew the tablets.
30 Tablets




