Glycol-3350
What is GoLYTELY (Glycol-3350)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this research is to compare patient preferences for two bowel preparation options: low-volume tablets (Suflave/Sutab) versus the standard colon preparation using Golytely (polyethylene glycol). The study aims to enroll approximately 300 patients, who will be randomly assigned to one of the two preparation methods in a 2:1 ratio prior to their scheduled colonoscopy appointments. Surv...
Summary: The proposed study will evaluate the safety and efficacy of XRT followed by systemic therapy among patients with HER2+ metastatic breast cancer and LMD
Summary: This study will evaluate changes in the fecal microbiome in constipated pediatric patients before and after antegrade continence enema placement and initiation of antegrade enema flushes. Subjects will have their microbiome sequenced prior to placement by obtaining a fecal sample. Pre-antegrade continence enema placement results will be compared to fecal samples obtained at 0, 4, 8 months after pl...
Related Latest Advances
Brand Information
- Gastrointestinal (GI) obstruction
- Bowel perforation
- Toxic colitis or toxic megacolon
- Gastric retention
- Ileus
- Hypersensitivity to any component of GoLYTELY
- Renal impairment
- Colonic mucosal ulcerations and ischemic colitis
- Patients with significant gastrointestinal disease
- Aspiration
- Cardiovascular: arrhythmia, atrial fibrillation, peripheral edema, asystole, and acute pulmonary edema after aspiration
- Nervous system: tremor, seizure
- Hypersensitivity: Urticaria/rash, pruritus, dermatitis, rhinorrhea, dyspnea, chest and throat tightness, fever, angioedema, anaphylaxis and anaphylactic shock
- Gastrointestinal: Nausea, abdominal fullness and bloating are the most common adverse reactions (occurred in up to 50% of patients). Other less common adverse reactions include: abdominal cramps, vomiting, “butterfly-like” infiltrates on chest X-ray after vomiting and aspirating PEG, anal irritation, and upper GI bleeding from Mallory-Weiss Tear, esophageal perforation [usually with gastroesophageal reflux disease (GERD)].
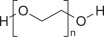
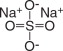
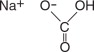
- When reconstituted with water to a volume of 4 liters, the solution contains 59 g/L PEG-3350, 5.69 g/L sodium sulfate, 1.69 g/L sodium bicarbonate, 1.47 g/L sodium chloride and 0.743 g/L potassium chloride.
- To reconstitute GoLYTELY with water prior to ingestion.
- Not to take other laxatives while they are taking GoLYTELY.
- Not to take oral medications within 1 hour before the start or during the administration of GoLYTELY.
- To take only clear liquids but avoid red and purple liquids.
- To consume water or other clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- To follow the directions in the
- If they experience severe bloating, distention or abdominal pain, to slow or temporarily discontinue drinking the solution and to contact their healthcare provider.
- To contact their healthcare provider if they develop signs and symptoms of dehydration or if they experience altered consciousness or seizures.
- To discontinue administration of the solution and contact their healthcare provider if they develop symptoms of a hypersensitivity reaction
