Vinorelbine
View Brand InformationWhat is Vinorelbine?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The hypothesize is that tepotinib is more effective than the investigator's choice of treatment in patients with MET-mutated NSCLC who have progressed after at least one first-line treatment. The main benefit concerns patient access to tepotinib. There is currently no access to a new-generation MET TKI in France for METex14 patients, due to lack of comparative data. There are no phase III RCTs und...
Summary: This phase III trial compares standard therapy given after surgery (adjuvant) to standard therapy given before and after surgery (perioperative) in treating patients with stage II-IIIB non-small cell lung cancer (NSCLC) that can be removed by surgery (resectable). The usual approach for patients with resectable NSCLC is chemotherapy and/or immunotherapy before surgery, after surgery, or both befor...
Summary: The main purpose of the master is to help the research sites and sponsor carry out several clinical trials more efficiently by providing a common research protocol. Individual clinical trials under this master protocol define drug/disease-specific research goals and activities to test them. New studies will be added as new drugs emerge against different cancers. Participation in the trial will dep...
Related Latest Advances
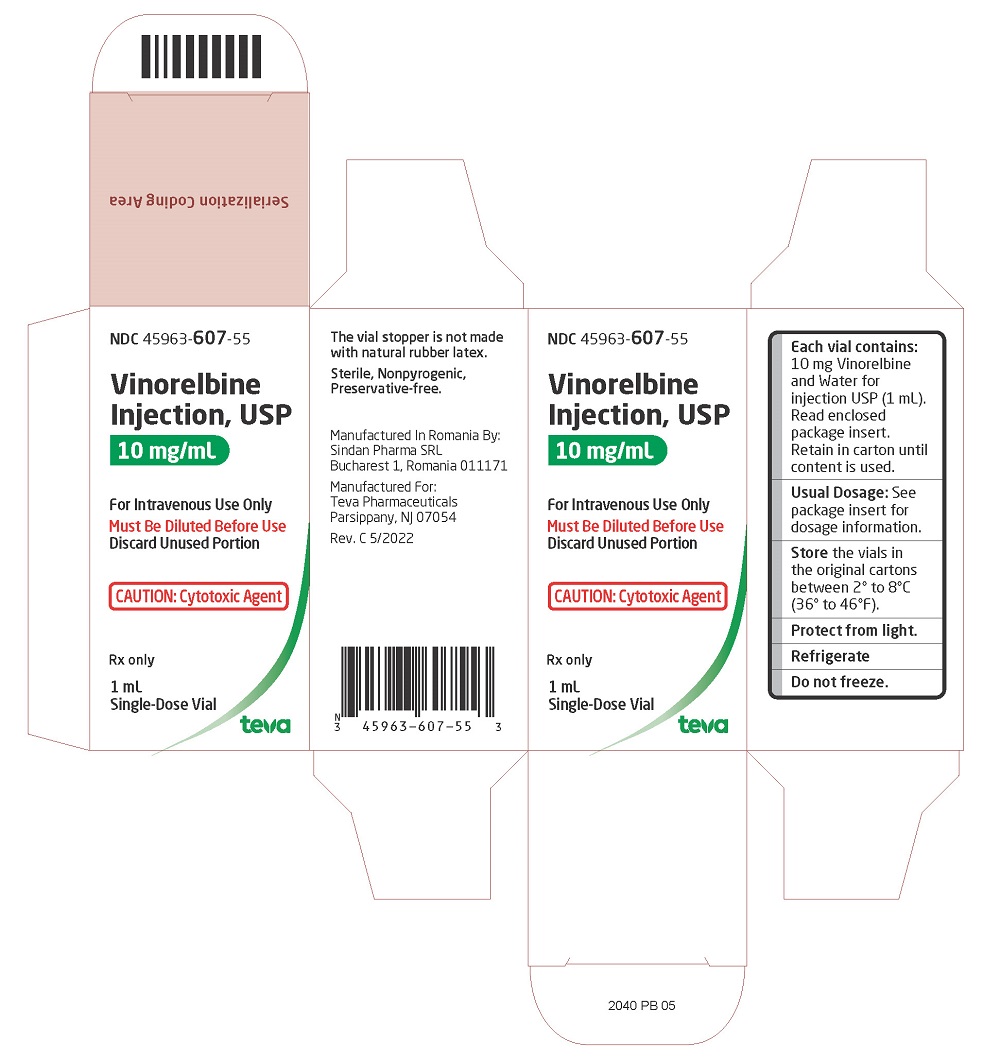
Brand Information
- Severe myelosuppression resulting in serious infection, septic shock, hospitalization and death can occur [see
- Decrease the dose or withhold vinorelbine in accord with recommended dose modifications [see
- In combination with cisplatin for first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC)
- As a single agent for the treatment of patients with metastatic NSCLC
- Myelosuppression
- Hepatic Toxicity
- Severe Constipation and Bowel Obstruction
- Extravasation and Tissue Injury
- Neurologic Toxicity
- Pulmonary Toxicity and Respiratory Failure
- OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
- Advise pregnant women of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during treatment with vinorelbine and for 6 months after the final dose
- Advise males with female partners of reproductive potential to use effective contraception during treatment with vinorelbine and for 3 months after the final dose