Generic Name
Tenapanor
Brand Names
Ibsrela, Xphozah
FDA approval date: September 12, 2019
Classification: Sodium-Hydrogen Exchanger 3 Inhibitor
Form: Tablet
What is Ibsrela (Tenapanor)?
IBSRELA is indicated for treatment of irritable bowel syndrome with constipation in adults. IBSRELA is a sodium/hydrogen exchanger 3 inhibitor indicated for treatment of irritable bowel syndrome with constipation in adults.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
IBSRELA (tenapanor hydrochloride)
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
- IBSRELA is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile rats administration of tenapanor caused deaths presumed to be due to dehydration
- Avoid use of IBSRELA in patients 6 years to less than 12 years of age
- The safety and effectiveness of IBSRELA have not been established in patients less than 18 years of age
1INDICATIONS AND USAGE
IBSRELA is indicated for treatment of irritable bowel syndrome with constipation (IBS-C) in adults.
2DOSAGE AND ADMINISTRATION
The recommended dosage of IBSRELA in adults is 50 mg orally twice daily.
3DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg tenapanor supplied as an oval, white to off-white tablet debossed with "
4CONTRAINDICATIONS
IBSRELA is contraindicated in:
- Patients less than 6 years of age due to the risk of serious dehydration
- Patients with known or suspected mechanical gastrointestinal obstruction
5OVERDOSAGE
Based on nonclinical data, overdose of IBSRELA may result in gastrointestinal adverse effects such as diarrhea as a result of exaggerated pharmacology with a risk for dehydration if diarrhea is severe or prolonged
6DESCRIPTION
IBSRELA (tenapanor) tablets contain tenapanor hydrochloride as an active ingredient. Tenapanor hydrochloride is a sodium/hydrogen exchanger 3 (NHE3) inhibitor for oral use. The chemical name for tenapanor hydrochloride is 12,15-Dioxa-2,7,9-triazaheptadecanamide, 17-[[[3-[(4S)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl]phenyl]sulphonyl]amino]-N-[2-[2-[2-[[[3-[(4S)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl]phenyl]sulphonyl]amino]ethoxy]ethoxy]ethyl]-8-oxo-, hydrochloride (1:2). Tenapanor hydrochloride has the molecular formula of C
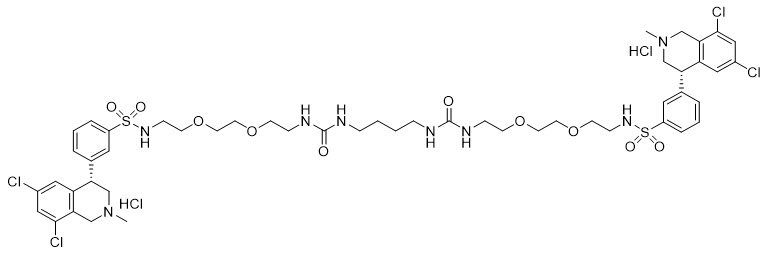
Tenapanor hydrochloride is a white to off-white to light brown hygroscopic amorphous solid. It is practically insoluble in water.
IBSRELA tablets contain 50 mg of tenapanor (equivalent to 53.2 mg of tenapanor hydrochloride). Inactive ingredients in the tablet are colloidal silicon dioxide, low-substituted hydroxypropyl cellulose, microcrystalline cellulose, propyl gallate, stearic acid, tartaric acid, and the coating agent OPADRY
7CLINICAL STUDIES
The efficacy of IBSRELA for the treatment of IBS-C was established in two double-blind, placebo-controlled, randomized, multicenter trials in adult patients: Trial 1 (TEN-01-302; NCT02686138) and Trial 2 (TEN-01-301; NCT02621892). The intent-to-treat (ITT) analysis population included 620 patients in Trial 1 and 606 patients in Trial 2 with mean age of 46 years (range 18 to 75 years), 80% females, 64% White and 31% Black/African American. In these clinical trials, IBSRELA was administered immediately prior to breakfast or the first meal of the day and immediately prior to dinner.
To enter the trials, all patients met Rome III criteria for IBS-C and were required to meet the following clinical criteria during the 2-week baseline run-in period:
- a mean abdominal pain score of at least 3 on a 0-to-10-point numeric rating scale where a score of 0 indicates no pain and 10 indicates very severe pain
- less than 3 complete spontaneous bowel movements (CSBMs) per week, where a CSBM is defined as a spontaneous bowel movement (SBM) that is associated with a sense of complete evacuation (an SBM is a bowel movement occurring in the absence of laxative use)
- less than or equal to 5 SBMs per week
The trial designs were identical through the first 12 weeks of treatment, and thereafter differed in that Trial 1 continued for an additional 14 weeks of treatment (26 weeks double-blind treatment), whereas Trial 2 included a 4-week randomized withdrawal (RW) period.
Efficacy of IBSRELA was assessed using responder analyses based on daily diary entries.
In both trials, the primary endpoint was the proportion of responders, where a responder was defined as a patient achieving both the stool frequency and abdominal pain intensity responder criteria in the same week for at least 6 of the first 12 weeks of treatment. The stool frequency (CSBM) and abdominal pain responder criteria assessed each week were defined as:
- CSBM responder: a patient who experienced an increase of at least 1 CSBM in weekly average from baseline.
- Abdominal pain responder: a patient who experienced at least a 30% reduction in the weekly average of abdominal pain score compared with baseline.
The responder rates for the primary endpoint and components of the primary endpoint (CSBM and abdominal pain), which were pre-specified key secondary endpoints, are shown in Table 2.
In Trials 1 and 2, the proportion of responders for 9 out of the first 12 weeks, including at least 3 of the last 4 weeks, was greater in IBSRELA-treated patients compared to placebo-treated patients. In addition, in Trial 1, the proportion of responders for 13 out of 26 weeks was greater in IBSRELA-treated patients compared to placebo-treated patients.
In both trials, improvements from baseline in average weekly CSBMs and abdominal pain were observed by Week 1, with improvement maintained through the end of treatment.
In IBSRELA-treated patients re-randomized to placebo in Trial 2, CSBM frequency and abdominal pain severity worsened on average over the 4-week period but remained improved compared to baseline. Patients who continued on IBSRELA maintained their response to therapy on average over the additional 4 weeks
8HOW SUPPLIED/STORAGE AND HANDLING
IBSRELA tablets contain 50 mg tenapanor and are oval, white to off-white, debossed with "
IBSRELA is supplied in a white, opaque, high-density polyethylene bottle containing 60 tablets with a silica gel canister (as the desiccant) and screw-top polypropylene child-resistant cap lined and induction-activated aluminum foil liner (NDC 73154-050-60).
9PATIENT COUNSELING INFORMATION
Advise the patients to read the FDA-approved patient labeling (Medication Guide).
10PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label - 050-60
NDC 73154-050-60
IBSRELA
50 mg
ATTENTION PHARMACIST:
Attention Pharmacist:
60 tablets

11PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label - 050-06
PROFESSIONAL SAMPLE – NOT FOR SALE
IBSRELA
50 mg
ATTENTION PHARMACIST: Dispense the
Attention Pharmacist:
6 tablets
