Tpoxx
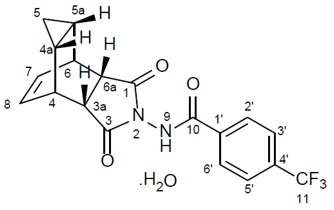
What is Tpoxx (Tecovirimat)?
Approved To Treat
Related Clinical Trials
Summary: The overall purpose of this study is to evaluate whether tecovirimat is an efficient and safe antiviral in the treatment of monkeypox in adults and adolescents (14 years old and older). The primary objective is to evaluate the clinical efficacy, as assessed by time to all visible lesion(s) resolution, of tecovirimat treatment + Standard of Care (SOC) compared to placebo + SOC for patients with mon...
Summary: The goal of this randomized controlled double-blind clinical trial is to test the drug tecovirimat in patients with mpox (previously known as monkeypox) disease. The main questions it aims to answer are: * Is tecovirimat effective in treating mpox infection. * Is tecovirimat safe to treat patients with mpox infection. Participants will receive either the drug tecovirimat orally, 600 mg twice per d...
Related Latest Advances
Brand Information