Brand Name
Seysara
Generic Name
Sarecycline
View Brand Information FDA approval date: January 04, 2019
Classification: Tetracycline-class Drug
Form: Tablet
What is Seysara (Sarecycline)?
SEYSARA ® tablet, is indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older. Limitations of Use Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established. SEYSARA has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, SEYSARA should be used only as indicated [see Warnings and Precautions.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Seysara (sarecycline hydrochloride)
1INDICATIONS AND USAGE
SEYSARA
Limitations of Use
Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established. SEYSARA has not been evaluated in the treatment of infections [see Clinical Studies (.
Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established. SEYSARA has not been evaluated in the treatment of infections [see Clinical Studies (.
To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, SEYSARA should be used only as indicated
2DOSAGE AND ADMINISTRATION
The recommended dosage of SEYSARA is based on body weight described in
Take SEYSARA once daily, with or without food. To reduce the risk of esophageal irritation and ulceration, administer SEYSARA with adequate amounts of fluid.
3DOSAGE FORMS AND STRENGTHS
SEYSARA (sarecycline) tablets:
- 60 mg: capsule-shaped, yellow, film-coated tablets debossed with “S60” on one side and blank on the other side.
- 100 mg: capsule-shaped, yellow, film-coated tablets debossed with “S100” on one side and blank on the other side.
- 150 mg: capsule-shaped, yellow, film-coated tablets debossed with “S150” on one side and blank on the other side.
4CONTRAINDICATIONS
SEYSARA is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
5OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases of overdose.
6DESCRIPTION
SEYSARA (sarecycline) tablets are a tetracycline class drug for oral administration. Sarecycline hydrochloride is chemically described as (4
The structural formula is represented below:
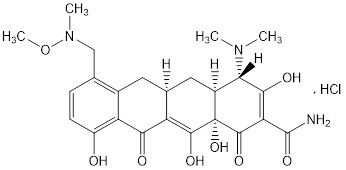
SEYSARA tablets contain 64.5 mg, 107.5 mg, and 161.2 mg of sarecycline hydrochloride equivalent to 60 mg, 100 mg, and 150 mg sarecycline respectively. Inactive ingredients in the tablet formulations are: microcrystalline cellulose, povidone, sodium starch glycolate, and sodium stearyl fumarate. The yellow film coating contains D&C yellow #10 aluminum lake, iron oxide yellow, methacrylic acid copolymer type C, polyethylene glycol, polyvinyl alcohol, sodium bicarbonate, talc, and titatnium dioxide.
7CLINICAL STUDIES
The safety and efficacy of once daily SEYSARA was assessed in two 12-week multicenter, randomized, double-blind, placebo-controlled studies (Study 1 [NCT02320149] and Study 2 [NCT02322866]). Efficacy was assessed in a total of 2002 subjects 9 years of age and older. Overall, 57% were female, 78% were Caucasian, 15% were Black or African American and 51% were adults (18 to 45 years of age). Subjects were randomized to receive either SEYSARA or placebo once daily.
The two co-primary efficacy endpoints were:
- Percentage of subjects with Investigator’s Global Assessment (IGA) success: a score of clear (0) or almost clear (1) and 2-point decrease from baseline on IGA score at Week 12.
- Absolute reduction from baseline in inflammatory lesion counts at Week 12.
The results at Week 12 are presented in the following table.
Mean absolute and percent reduction in inflammatory lesions was also greater with SEYSARA compared to placebo at Weeks 3, 6, and 9 for both studies.
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- SEYSARA (sarecycline) tablets, 60 mg are capsule-shaped, yellow, film-coated tablets debossed with “S60” on one side and blank on the other side.
- SEYSARA (sarecycline) tablets, 100 mg are capsule-shaped, yellow, film-coated tablets debossed with “S100” on one side and blank on the other side.
- SEYSARA (sarecycline) tablets, 150 mg are capsule-shaped, yellow, film-coated tablets debossed with “S150” on one side and blank on the other side.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Patients taking SEYSARA should receive the following information and instructions:
- SEYSARA should not be used by pregnant women or women attempting to conceive a child
- Advise a woman that breastfeeding is not recommended during SEYSARA therapy.
- Advise patients that
- Advise patients that intracranial hypertension can occur with tetracycline therapy. If patients experience headache or blurred vision, they should seek medical attention.
- Patients who experience central nervous system symptoms should be cautioned about driving vehicles or using hazardous machinery while on SEYSARA therapy. Patients should seek medical help for persistent central nervous system symptoms.
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Advise patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using SEYSARA. If patients need to be outdoors while using SEYSARA, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Treatment should be discontinued at the first evidence of skin erythema.
- Advise patients that because of the potential for drug-resistant bacteria to develop during the use of SEYSARA, take SEYSARA as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the current treatment course and increase the likelihood that bacteria will develop resistance and will not be treatable by other antibacterial drugs in the future.
- Advise patients to drink fluids liberally along with SEYSARA to reduce the risk of esophageal irritation and ulceration
© 2019 Almirall, LLC. All rights reserved.
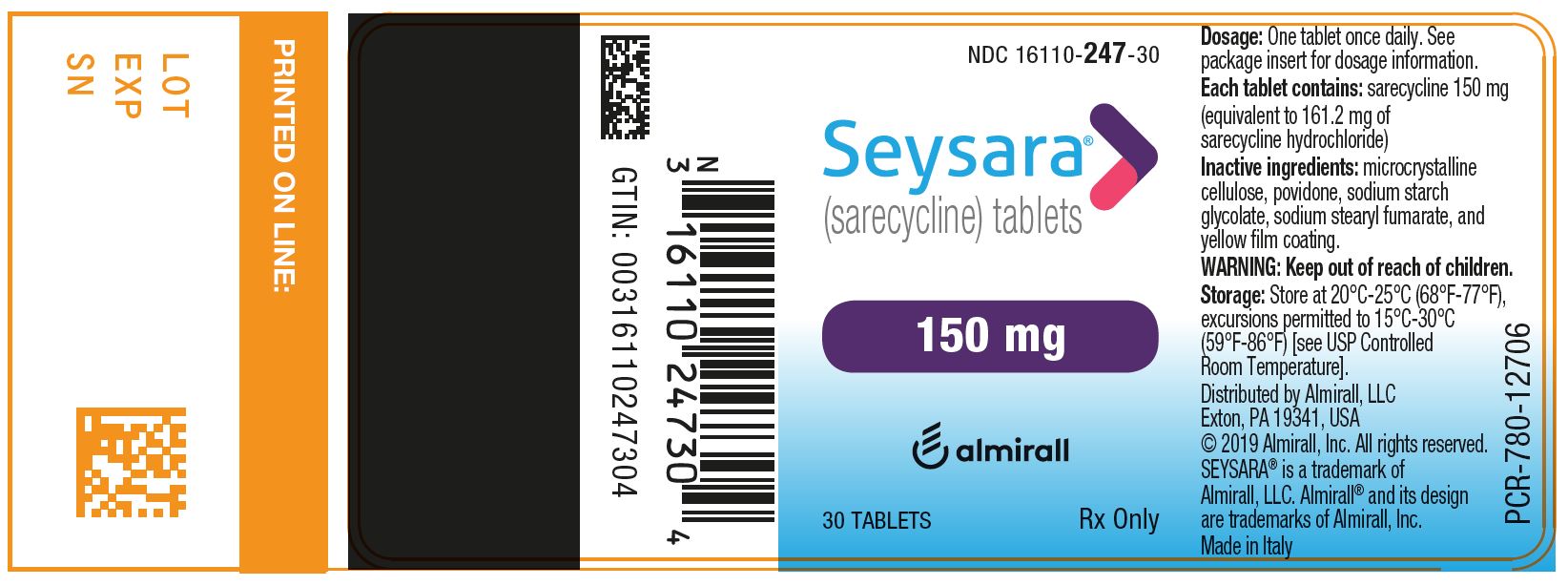
10PRINCIPAL DISPLAY PANEL - NDC: 16110-245-30 - 60 mg 30-count Bottle Label
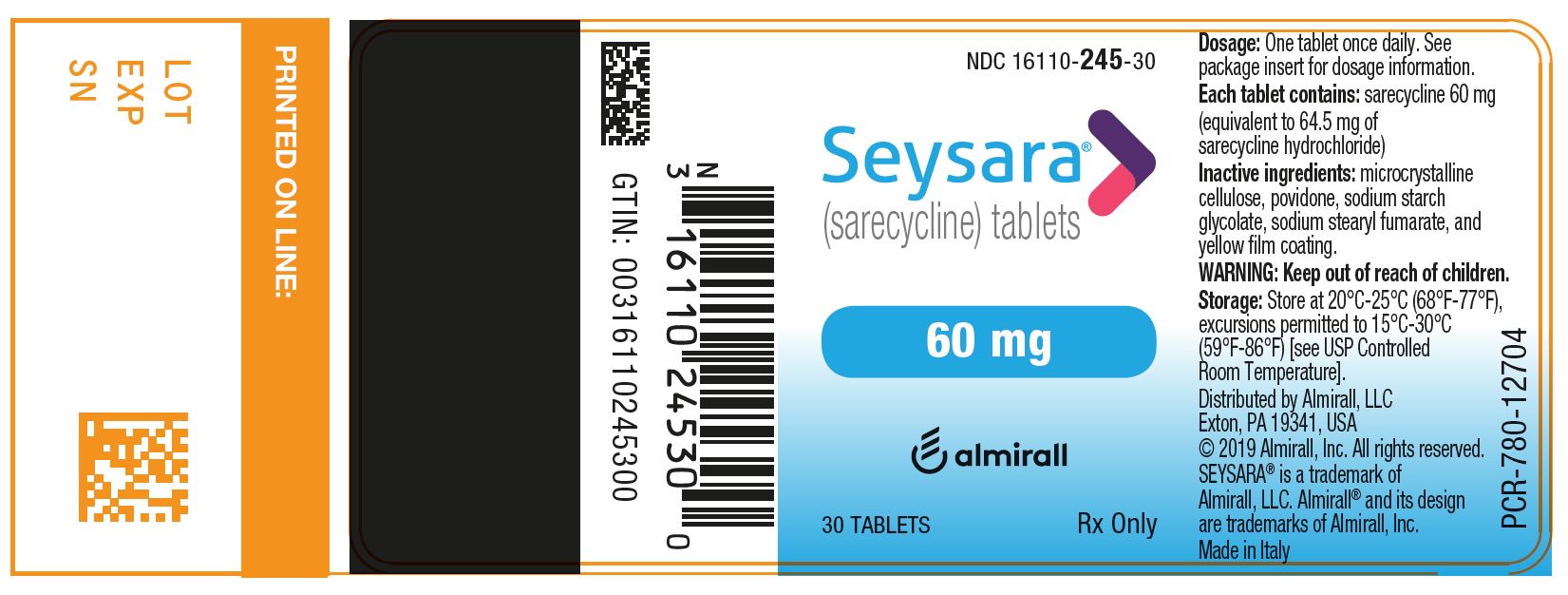
11PRINCIPAL DISPLAY PANEL - NDC: 16110-246-30 - 100 mg 30-count Bottle Label
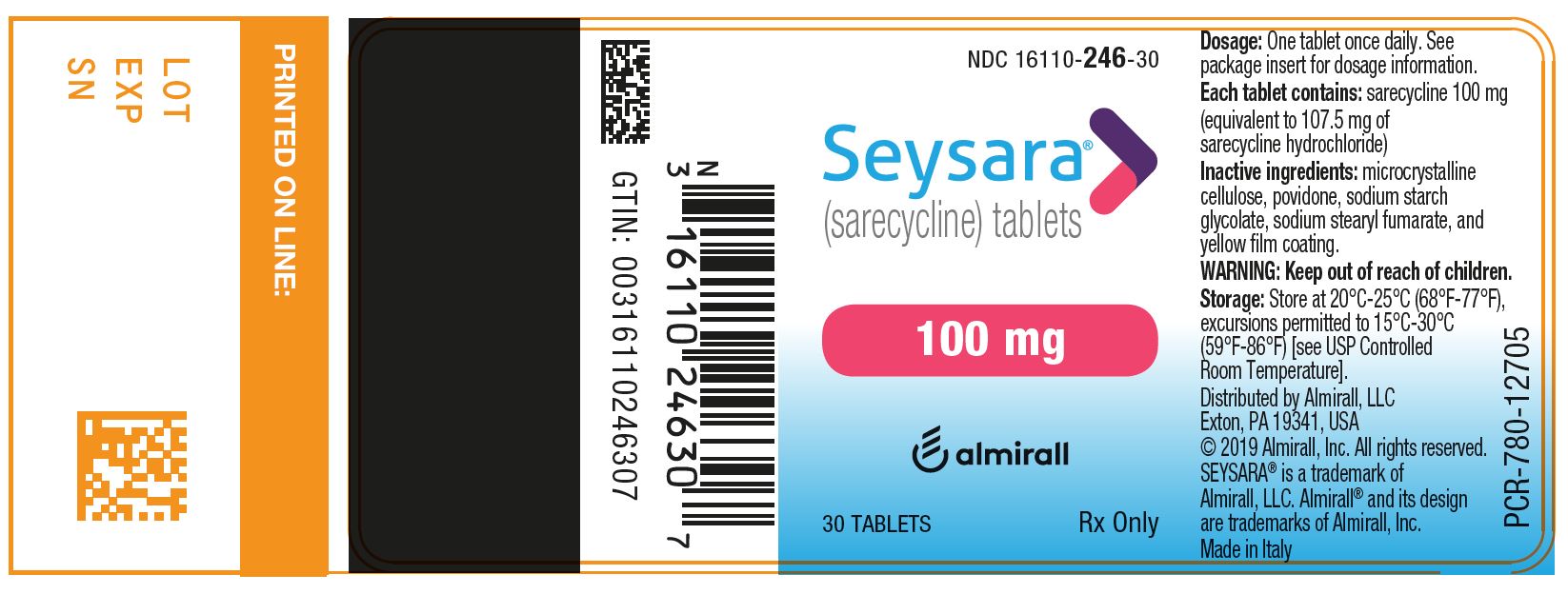
12PRINCIPAL DISPLAY PANEL - NDC: 16110-247-30 - 150 mg 30-count Bottle Label