Brand Name
Dipentum
Generic Name
Olsalazine
View Brand Information FDA approval date: May 15, 2015
Classification: Aminosalicylate
Form: Capsule
What is Dipentum (Olsalazine)?
DIPENTUM is indicated for the maintenance of remission of ulcerative colitis in adult patients who are intolerant of sulfasalazine.
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Dipentum (olsalazine sodium)
1INDICATIONS AND USAGE
DIPENTUM is indicated for the maintenance of remission of ulcerative colitis in adult patients who are intolerant of sulfasalazine.
2DOSAGE AND ADMINISTRATION
Evaluate renal function before initiating therapy with DIPENTUM
The recommended dosage is 500 mg orally twice daily.
Drink an adequate amount of fluids during treatment
3DOSAGE FORMS AND STRENGTHS
Capsules: 250 mg olsalazine sodium in beige capsules imprinted with DIPENTUM® 250 mg on the capsule shell
4CONTRAINDICATIONS
DIPENTUM is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the excipients in DIPENTUM
5ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Renal impairment
- Mesalamine-induced acute intolerance syndrome
- Hypersensitivity reactions
- Hepatic failure
- Severe cutaneous adverse reactions
- Photosensitivity
- Nephrolithiasis
The following adverse reactions have been identified from clinical studies or postmarketing reports of olsalazine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In double-blind, placebo- and active-controlled clinical trials of ulcerative colitis, discontinuations due to adverse reactions were reported in 10% of DIPENTUM-treated patients (N=441) and 7% of placebo-treated patients (N=208). Both sulfasalazine-tolerant and intolerant patients were included. The most common adverse reactions leading to discontinuation in DIPENTUM-treated patients were diarrhea/loose stools (6%), abdominal pain (1%), and rash/itching (1%).
In these controlled trials, adverse reactions reported in 1% or more of patients treated with DIPENTUM and greater than placebo are provided in Table 1.
Other adverse reactions reported in clinical trials or post-marketing experience:
Blood and Lymphatic System Disordersaplastic anemia, anemia, eosinophilia, hemolytic anemia, leukopenia, lymphopenia, neutropenia, pancytopenia, reticulocytosis, thrombocytopenia
Cardiac Disorderschest pains, heart block second degree, myocarditis, palpitations, pericarditis, peripheral edema, shortness of breath, tachycardia
A patient who developed thyroid disease 9 days after starting DIPENTUM was given propranolol and radioactive iodine and subsequently developed shortness of breath and nausea. The patient died 5 days later with signs and symptoms of acute diffuse myocarditis.
Ear and Labyrinth Disorderstinnitus
Eye Disordersdry eyes, vision blurred, watery eyes
Gastrointestinal Disorders
abdominal pain (upper), diarrhea with dehydration, dry mouth, epigastric discomfort, flare in symptoms, flatulence, increased blood in stool, pancreatitis, rectal bleeding, rectal discomfort
abdominal pain (upper), diarrhea with dehydration, dry mouth, epigastric discomfort, flare in symptoms, flatulence, increased blood in stool, pancreatitis, rectal bleeding, rectal discomfort
General Disorders and Administration Site Conditions
fever chills, hot flashes, irritability, pyrexia, rigors
fever chills, hot flashes, irritability, pyrexia, rigors
Hepatobiliary Disordershepatic enzyme increased, hepatitis (including cholestasis, granulomatous, and non-specific, reactive), increased bilirubin
Reports of hepatotoxicity, including elevated liver function tests (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. One case of Kawasaki-like syndrome, which included hepatic function changes, was also reported.
Immune System Disordersbronchospasm, erythema nodosum
Musculoskeletal and Connective Tissue Disordersmyalgia, muscle cramps
Nervous System Disordersinsomnia, paraesthesia, peripheral neuropathy, tremors
Psychiatric Disordersmood swings
Renal and Urinary Disordersdysuria, hematuria, interstitial nephritis, nephrolithiasis, nephrotic syndrome, proteinuria, urinary frequency
- Urine discoloration occurring
Reproductive System and Breast Disorders
impotence, menorrhagia, reversible oligospermia
impotence, menorrhagia, reversible oligospermia
Respiratory, Thoracic and Mediastinal Disordersdyspnea, interstitial lung disease, pleurisy/pleuritis
Skin and Subcutaneous Tissue DisordersAGEP, alopecia, angioneurotic edema, DRESS, erythema, photosensitivity reaction, SJS/TEN
Vascular Disordershypertension, orthostatic hypotension
6OVERDOSAGE
DIPENTUM is an aminosalicylate, and symptoms of salicylate toxicity include: nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe salicylate intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) damage.
There is no specific antidote for olsalazine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent of further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
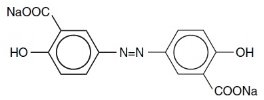
7DESCRIPTION
The active ingredient in DIPENTUM (olsalazine sodium) is the sodium salt of a salicylate, disodium 3,3'-azobis (6-hydroxybenzoate) a compound that is effectively bioconverted to mesalamine (5-aminosalicylic acid,5-ASA), an aminosalicylate. Its empirical formula is C14H8N2Na2O6 with a molecular weight of 346.21.
The structural formula is:
Olsalazine sodium is a yellow crystalline powder, which melts with decomposition at 240°C. It is the sodium salt of a weak acid, soluble in water and DMSO, and practically insoluble in ethanol, chloroform, and ether. Olsalazine sodium has acceptable stability under acidic or basic conditions.
DIPENTUM is supplied in capsules for oral administration. Each DIPENTUM hard gelatin capsule contains 250 mg olsalazine sodium (equivalent to 233.4 mg of olsalazine). The inert ingredient in each capsule is magnesium stearate. The capsule shell contains the following inactive ingredients: black iron oxide, caramel, gelatin, and titanium dioxide.
8CLINICAL STUDIES
Two double-blind controlled trials have demonstrated the efficacy of DIPENTUM as maintenance therapy in patients with ulcerative colitis. In the first trial, patients with ulcerative colitis in remission were randomized to DIPTENUM 500 mg twice daily or placebo, and relapse rates for a six month period of time were compared. For the 52 patients randomized to DIPENTUM, 12 relapses occurred, while for the 49 patients randomized to placebo, 22 relapses occurred. This difference in relapse rates was significant (p<0.02).
In the second trial, 164 patients with ulcerative colitis in remission were randomized to DIPENTUM 500 mg twice daily or sulfasalazine 1 gram twice daily, and relapse rates were compared after six months. The relapse rate for DIPENTUM-treated patients was 19.5% and 12.2% for sulfasalazine-treated patients, a non-significant difference.
9HOW SUPPLIED/STORAGE AND HANDLING
DIPENTUM is supplied as beige colored capsules, containing 250 mg olsalazine sodium imprinted with “DIPENTUM
Bottles of 100’s NDC 0037-6860-10
Store at 20°C to 25°C (68°F to 77°F). Excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
10PATIENT COUNSELING INFORMATION
Renal Impairment
Inform patients that DIPENTUM may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, and periodic monitoring of renal function will be performed while they are on therapy. Advise patients to complete all blood tests ordered by their healthcare provider
Mesalamine-Induced Acute Intolerance Syndrome and Other Hypersensitivity Reactions
Instruct patients to stop taking DIPENTUM and report to their healthcare provider if they experience new or worsening symptoms of acute intolerance syndrome (cramping, abdominal pain, bloody diarrhea, fever, headache, and rash) or other symptoms suggestive of mesalamine-induced hypersensitivity
Hepatic Failure
Advise patients with known liver disease to contact their healthcare provider if they experience signs or symptoms of worsening liver function
Severe Cutaneous Adverse Reactions
Inform patients of the signs and symptoms of severe cutaneous adverse reactions. Instruct patients to stop taking DIPENTUM and report to their healthcare provider at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity
Photosensitivity
Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors
Nephrolithiasis
Instruct patients to drink an adequate amount of fluids during treatment in order to minimize the risk of kidney stone formation and to contact their healthcare provider if they experience signs or symptoms of a kidney stone (e.g., severe side or back pain, blood in the urine)
Blood Disorders
Inform elderly patients and those taking azathioprine or 6-mercaptopurine of the risk for blood disorders and the need for periodic monitoring of complete blood cell counts and platelet counts while on therapy. Advise patients to complete all blood tests ordered by their healthcare provider
Administration
- Instruct patients to drink an adequate amount of fluids during treatment.
- Advise patients that urine may become discolored reddish-brown while taking DIPENTUM when it comes in contact with surfaces or water treated with hypochlorite-containing bleach. If discolored urine is observed, advise patients to observe their urine flow. Report to the healthcare provider only if urine is discolored on leaving the body, before contact with any surface or water (e.g., in the toilet)
Manufactured for:
Manufactured by:
© 2024 Viatris Inc.
DIPENTUM is a registered trademark of Alaven Pharmaceutical LLC, a Viatris Company.
Rev. 7/2024
11PRINCIPAL DISPLAY PANEL – 250 mg
NDC 0037-6860-10
Dipentum®(olsalazine sodium)
capsule, gelatin coated
capsule, gelatin coated
250 mg
Rx only
100 Capsules
USUAL DOSAGE: See package circular
for complete product information.
for complete product information.
PHARMACIST: Dispense in a
well-closed container.
well-closed container.
Store at 25°C (77°F); excursions
Manufactured for:
Manufactured by:
©2023 Viatris Inc.
DIPENTUM is a registered trademark
LB-686010-05