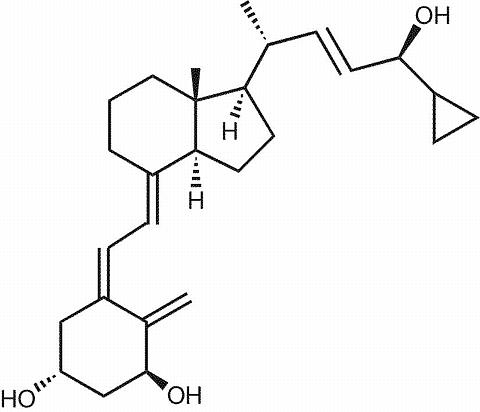
Calcipotriene
What is Trionex (Calcipotriene)?
Approved To Treat
Related Clinical Trials
Summary: The goal of this clinical research study is to compare the effects of topical fluorouracil alone to topical fluorouracil plus topical calcipotriene in patients with multiple actinic keratoses. Topical means the medication is applied directly to the skin.
Summary: This clinical trial proposes to evaluate a relatively unexplored approach to treatment of squamous cell carcinoma (SCC) on the lower extremities. The strategy is to directly and specifically deliver drug to the tumor. For the proposed phase I clinical trial, the investigators will perform intralesional injections of a well characterized, potent chemotherapeutic agent 5-fluorouracil (5FU) with and ...
Summary: The investigators will compare the application of two different creams for the treatment of low-risk skin cancers-superficial basal cell carcinoma (sBCC) and squamous cell carcinoma in situ (SCCis). 5-Fluorouracil cream is currently FDA approved for the treatment of superficial basal cell carcinoma and is routinely used by dermatologists across the country and at Boston Medical Center (BMC) for SC...
Related Latest Advances
Brand Information