Brand Name
Evenity
Generic Name
Romosozumab-Aqqg
View Brand Information FDA approval date: April 09, 2019
Form: Injection
What is Evenity (Romosozumab-Aqqg)?
EVENITY is a sclerostin inhibitor indicated for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy. Limitations of Use: Limit duration of use to 12 monthly doses. If osteoporosis therapy remains warranted, continued therapy with an anti-resorptive agent should be considered.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Evenity (romosozumab-aqqg)
WARNING: POTENTIAL RISK OF MYOCARDIAL INFARCTION, STROKE AND CARDIOVASCULAR DEATH
- EVENITY may increase the risk of myocardial infarction, stroke, and cardiovascular death
1DOSAGE FORMS AND STRENGTHS
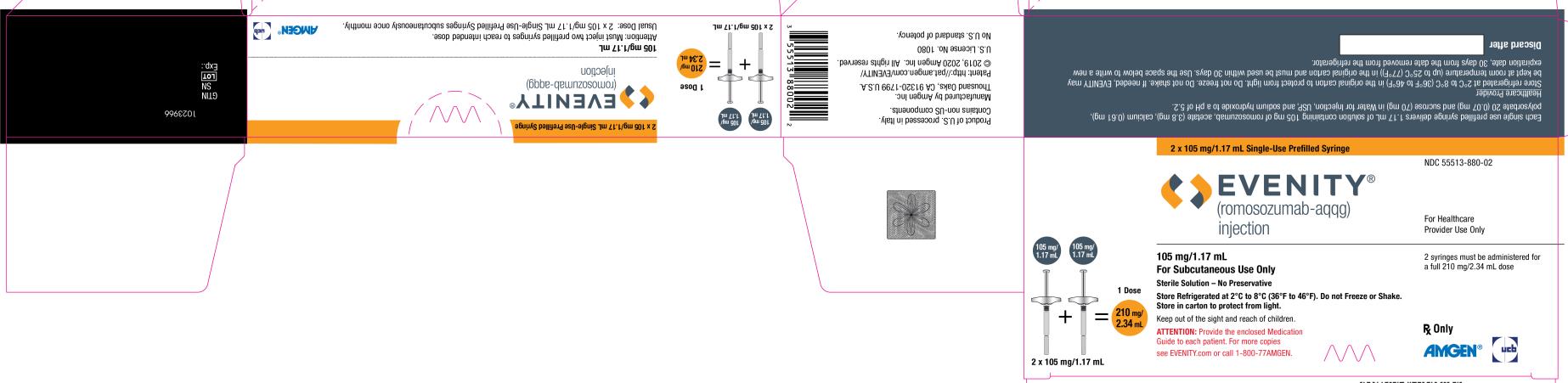
Injection: 105 mg/1.17 mL clear to opalescent, colorless to light yellow solution in a single-use prefilled syringe.
A full dose of EVENITY requires two single-use prefilled syringes.
2CONTRAINDICATIONS
EVENITY is contraindicated in patients with:
- Hypocalcemia. Pre-existing hypocalcemia must be corrected prior to initiating therapy with EVENITY
- A history of systemic hypersensitivity to romosozumab or to any component of the product formulation. Reactions have included angioedema, erythema multiforme, and urticaria
3ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Major adverse cardiac events
- Hypersensitivity
- Hypocalcemia
- Osteonecrosis of the Jaw
- Atypical Subtrochanteric and Diaphyseal Femoral Fractures
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of EVENITY for the treatment of postmenopausal osteoporosis was evaluated in a multicenter, randomized, double-blind, placebo-controlled study (Study 1, NCT01575834) of 7180 postmenopausal women aged 55 to 90 years (mean age of 71 years). A total of 3581 and 3576 women received at least one dose of EVENITY and placebo, respectively, administered once every month during the 12-month double-blind study period. Women received at least 500 mg calcium and 600 international units of vitamin D supplementation daily and 77% received a loading dose of 50,000 to 60,000 international units of vitamin D within one week of randomization (if serum 25-hydroxyvitamin D concentrations were 40 ng/mL or less).
The safety of EVENITY for the treatment of postmenopausal osteoporosis in patients at high risk of fracture was evaluated in a multicenter, randomized, double-blind, alendronate-controlled study (Study 2, NCT01631214) of 4093 postmenopausal women aged 55 to 90 years (mean age of 74 years). A total of 2040 and 2014 women received at least one dose of EVENITY and alendronate, respectively, during the 12-month double-blind study period. Women received at least 500 mg calcium and 600 international units vitamin D supplementation daily and 74% received a loading dose of 50,000 to 60,000 international units of vitamin D within one week of randomization (if serum 25-hydroxyvitamin D concentrations were 40 ng/mL or less).
In Study 1, during the 12-month double-blind treatment period, the incidence of all-cause mortality was 0.7% (24/3576) in the placebo group and 0.8% (29/3581) in the EVENITY group. The incidence of nonfatal serious adverse events was 8.3% in the placebo group and 9.1% in the EVENITY group. The percentage of patients who withdrew from the study due to adverse events was 1.1% in the placebo group and 1.1% in the EVENITY group. The most common adverse reactions reported with EVENITY (greater than or equal to 5% and at a higher incidence than placebo) were arthralgia and headache. The most common adverse reaction leading to discontinuation of EVENITY was arthralgia (6 subjects [0.2%] in the placebo group and 5 subjects [0.1%] in the EVENITY group).
In Study 2, during the 12-month double-blind treatment period, the incidence of all-cause mortality was 1.1% (22/2014) in the alendronate group and 1.5% (30/2040) in the EVENITY group. The incidence of nonfatal serious adverse events was 13.3% in the alendronate group and 11.9% in the EVENITY group. The percentage of patients who withdrew from the study due to adverse events was 1.2% in the alendronate group and 1.2% in the EVENITY group. The most common adverse reactions reported with EVENITY (greater than or equal to 5%) were arthralgia and headache.
Table 1 outlines the most common adverse reactions occurring in greater than or equal to 2% of EVENITY treated women in at least one study.
The adverse reactions described below are from the 12-month treatment periods of Study 1 (placebo-controlled) and Study 2 (alendronate-controlled).
3.2Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other romosozumab products may be misleading.
The immunogenicity of EVENITY was evaluated using an immunoassay for the detection of anti-romosozumab-aqqg antibodies. An
Among 5914 postmenopausal women treated with EVENITY 210 mg monthly, 18.1% of subjects developed antibodies to romosozumab-aqqg. Of the subjects who developed antibodies to romosozumab-aqqg, 4.7% had antibodies that were classified as neutralizing. Development of antibodies to romosozumab-aqqg was associated with lower serum romosozumab-aqqg concentrations
4DESCRIPTION
Romosozumab-aqqg is a humanized monoclonal antibody (IgG2) produced in a mammalian cell line (Chinese Hamster Ovary) by recombinant DNA technology that binds to and inhibits sclerostin. Romosozumab-aqqg has an approximate molecular weight of 149 kDa.
EVENITY (romosozumab-aqqg) injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to light yellow solution for subcutaneous injection in a single-use prefilled syringe.
Two 105 mg/1.17 mL single-use prefilled syringes are required to administer the recommended 210 mg dose of EVENITY
5PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
6PRINCIPAL DISPLAY PANEL - 105 mg/1.17 mL Syringe Carton
2 x 105 mg/1.17 mL Single-Use Prefilled Syringe
NDC 55513-998-02
EVENITY
For Healthcare
105 mg/
210 mg/
2 x 105 mg/1.17 mL
105 mg/1.17 mL
Sterile Solution – No Preservative
Store Refrigerated at 2°C to 8°C (36°F to 46°F). Do not Freeze or Shake.
Keep out of the sight and reach of children.
ATTENTION: Provide the enclosed Medication
see EVENITY.com or call 1-800-77AMGEN.
2 syringes must be administered for
Rx Only
AMGEN ucb