Brand Name
Dapzura
Generic Name
Daptomycin
View Brand Information FDA approval date: February 22, 2017
Classification: Lipopeptide Antibacterial
Form: Injection
What is Dapzura (Daptomycin)?
Staphylococcus aureus Bloodstream Infections , in Adult Patients, Including Those with Right-Sided Infective Endocarditis, Caused by Methicillin-Susceptible and Methicillin-Resistant Isolates Daptomycin for Injection is indicated for the treatment of adult patients with Staphylococcus aureus bloodstream infections , including adult patients with right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates. Pediatric use information is approved for Merck & Co., Inc.’s Cubicin . However, due to Merck & Co., Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information. Complicated Skin and Skin Structure Infections Daptomycin for Injection is indicated for the treatment of adult patients with complicated skin and skin structure infections caused by susceptible isolates of the following Gram-positive bacteria: Staphylococcus aureus , Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis, and Enterococcus faecalis . Pediatric use information is approved for Merck & Co., Inc.’s Cubicin . However, due to Merck & Co., Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information. Daptomycin for Injection is a lipopeptide antibacterial indicated for the treatment of: Complicated skin and skin structure infections in adult patients. Limitations of Use Daptomycin for Injection is not indicated for the treatment of pneumonia. Daptomycin for Injection is not indicated for the treatment of left-sided infective endocarditis due to S.aureus. The clinical trial of Daptomycin for Injection in adult patients with S. aureus bloodstream infections included limited data from patients with left-sided infective endocarditis; outcomes in these patients were poor [ see Clinical Studies ( 1. Appropriate specimens for microbiological examination should be obtained in order to isolate and identify the causative pathogens and to determine their susceptibility to daptomycin. To reduce the development of drug-resistant bacteria and maintain the effectiveness of Daptomycin for Injection and other antibacterial drugs, Daptomycin for Injection should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information is available, it should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Empiric therapy may be initiated while awaiting test results.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
DAPZURA RT (daptomycin)
1DOSAGE FORMS AND STRENGTHS
For Injection: 500 mg daptomycin as a sterile, pale yellow to light brown lyophilized powder for reconstitution in a single-dose vial.
2CONTRAINDICATIONS
DAPZURA RT is contraindicated in:
• Patients with known hypersensitivity to daptomycin
- • Patients with known or suspected Hereditary Fructose Intolerance (HFI)
3ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections:
- Anaphylaxis/Hypersensitivity Reactions
- Myopathy and Rhabdomyolysis
- Eosinophilic Pneumonia
- Drug Reaction with Eosinophilia and Systemic Symptoms
- Tubulointerstitial Nephritis
- Peripheral Neuropathy
- Increased International Normalized Ratio (INR)/Prolonged Prothrombin Time
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Adult Patients
Clinical trials enrolled 1,864 adult patients treated with daptomycin for injection and 1,416 treated with comparator.
Complicated Skin and Skin Structure Infection Trials in Adults
In Phase 3 complicated skin and skin structure infection (cSSSI) trials in adult patients, daptomycin for injection was discontinued in 15/534 (2.8%) patients due to an adverse reaction, while comparator was discontinued in 17/558 (3.0%) patients.
The rates of the most common adverse reactions, organized by body system, observed in adult patients with cSSSI (receiving 4 mg/kg daptomycin for injection) are displayed in
Drug-related adverse reactions (possibly or probably drug-related) that occurred in <1% of adult patients receiving daptomycin for injection in the cSSSI trials are as follows:
Body as a Whole: fatigue, weakness, rigors, flushing, hypersensitivity
Blood/Lymphatic System: leukocytosis, thrombocytopenia, thrombocytosis, eosinophilia, increased International Normalized Ratio (INR)
Cardiovascular System: supraventricular arrhythmia
Dermatologic System: eczema
Digestive System: abdominal distension, stomatitis, jaundice, increased serum lactate dehydrogenase
Metabolic/Nutritional System: hypomagnesemia, increased serum bicarbonate, electrolyte disturbance
Musculoskeletal System: myalgia, muscle cramps, muscle weakness, arthralgia
Nervous System: vertigo, mental status change, paresthesia
Special Senses: taste disturbance, eye irritation
- S. aureus Bacteremia/Endocarditis Trial in Adults
In the
Serious Gram-negative infections (including bloodstream infections) were reported in 10/120 (8.3%) daptomycin for injection-treated patients and 0/115 comparator-treated patients. Comparator-treated patients received dual therapy that included initial gentamicin for 4 days. Infections were reported during treatment and during early and late follow-up. Gram-negative infections included cholangitis, alcoholic pancreatitis, sternal osteomyelitis/mediastinitis, bowel infarction, recurrent Crohn’s disease, recurrent line sepsis, and recurrent urosepsis caused by a number of different Gram-negative bacteria.
The rates of the most common adverse reactions, organized by System Organ Class (SOC), observed in adult patients with
The following reactions, not included above, were reported as possibly or probably drug-related in the daptomycin for injection-treated group:
Blood and Lymphatic System Disorders: eosinophilia, lymphadenopathy, thrombocythemia, thrombocytopenia
Cardiac Disorders: atrial fibrillation, atrial flutter, cardiac arrest
Ear and Labyrinth Disorders: tinnitus
Eye Disorders: vision blurred
Gastrointestinal Disorders: dry mouth, epigastric discomfort, gingival pain, hypoesthesia oral
Infections and Infestations: candidal infection NOS, vaginal candidiasis, fungemia, oral candidiasis, urinary tract infection fungal
Investigations: blood phosphorous increased, blood alkaline phosphatase increased, INR increased, liver function test abnormal, alanine aminotransferase increased, aspartate aminotransferase increased, prothrombin time prolonged
Metabolism and Nutrition Disorders: appetite decreased NOS
Musculoskeletal and Connective Tissue Disorders: myalgia
Nervous System Disorders: dyskinesia, paresthesia
Psychiatric Disorders: hallucination NOS
Renal and Urinary Disorders: proteinuria, renal impairment NOS
Skin and Subcutaneous Tissue Disorders: pruritus generalized, rash vesicular
Other Trials in Adults
In Phase 3 trials of community-acquired pneumonia (CAP) in adult patients, the death rate and rates of serious cardiorespiratory adverse events were higher in daptomycin for injection-treated patients than in comparator-treated patients. These differences were due to lack of therapeutic effectiveness of daptomycin for injection in the treatment of CAP in patients experiencing these adverse events
Laboratory Changes in Adults
Complicated Skin and Skin Structure Infection Trials in Adults
In Phase 3 cSSSI trials of adult patients receiving daptomycin for injection at a dose of 4 mg/kg, elevations in CPK were reported as clinical adverse events in 15/534 (2.8%) daptomycin for injection-treated patients, compared with 10/558 (1.8%) comparator-treated patients. Of the 534 patients treated with daptomycin for injection, 1 (0.2%) had symptoms of muscle pain or weakness associated with CPK elevations to greater than 4 times the upper limit of normal (ULN). The symptoms resolved within 3 days and CPK returned to normal within 7 to 10 days after treatment was discontinued
S. aureus Bacteremia/Endocarditis Trial in Adults
In the
Clinical Trial Experience in Pediatric Patients
Complicated Skin and Skin Structure Infection Trial in Pediatric Patients
The safety of daptomycin for injection was evaluated in one clinical trial (in cSSSI), which included 256 pediatric patients (1 to 17 years of age) treated with intravenous daptomycin for injection and 133 patients treated with comparator agents. Patients were given age-dependent doses once daily for a treatment period of up to 14 days (median treatment period was 3 days). The doses given by age group were as follows: 10mg/kg for 1 to < 2 years, 9 mg/kg for 2 to 6 years, 7mg/kg for 7 to 11 years and 5 mg/kg for 12 to 17 years of age
Adverse Reactions Leading to Discontinuation
In the cSSSI study, daptomycin for injection was discontinued in 7/256 (2.7%) patients due to an adverse reaction, while comparator was discontinued in 7/133 (5.3%) patients.
Most Common Adverse Reactions
The rates of the most common adverse reactions, organized by body system, observed in these pediatric patients with cSSSI are displayed in
The safety profile in the clinical trial of cSSSI pediatric patients was similar to that observed in the cSSSI adult patients.
S. aureus Bacteremia Trial in Pediatric Patients
The safety of daptomycin for injection was evaluated in one clinical trial (in
Adverse Reactions Leading to Discontinuation
In the bacteremia study, daptomycin for injection was discontinued in 3/55 (5.5%) patients due to an adverse reaction, while comparator was discontinued in 2/26 (7.7%) patients.
Most Common Adverse Reactions
The rates of the most common adverse reactions, organized by body system, observed in these pediatric patients with bacteremia are displayed in
3.2Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of daptomycin for injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: anemia, thrombocytopenia
General and administration site conditions: pyrexia
Immune System Disorders: anaphylaxis; hypersensitivity reactions, including angioedema, pruritus, hives, shortness of breath, difficulty swallowing, truncal erythema, and pulmonary eosinophilia [see
Infections and Infestations: Clostridioides difficile–associated diarrhea [see Warnings and Precautions (5.8)]
Laboratory Investigations: platelet count decreased
Musculoskeletal Disorders: myoglobin increased; rhabdomyolysis (some reports involved patients treated concurrently with daptomycin for injection and HMG-CoA reductase inhibitors) [see
Respiratory, Thoracic, and Mediastinal Disorders: cough, eosinophilic pneumonia, organizing pneumonia [see
Skin and Subcutaneous Tissue Disorders: serious skin reactions, including drug reaction with eosinophilia and systemic symptoms (DRESS), vesiculobullous rash (with or without mucous membrane involvement, including Stevens-Johnson syndrome [SJS] and toxic epidermal necrolysis [TEN]), acute generalized exanthematous pustulosis [see
Gastrointestinal Disorders: nausea, vomiting
Renal and urinary disorders: acute kidney injury, renal insufficiency, renal failure, and tubulointerstitial nephritis (TIN) [see
Special Senses: visual disturbances
4OVERDOSAGE
In the event of overdosage, supportive care is advised with maintenance of glomerular filtration. Daptomycin is cleared slowly from the body by hemodialysis (approximately 15% of the administered dose is removed over 4 hours) and by peritoneal dialysis (approximately 11% of the administered dose is removed over 48 hours). The use of high-flux dialysis membranes during 4 hours of hemodialysis may increase the percentage of dose removed compared with that removed by low-flux membranes.
5DESCRIPTION
DAPZURA RT (daptomycin for injection) contains daptomycin, a cyclic lipopeptide antibacterial agent derived from the fermentation of
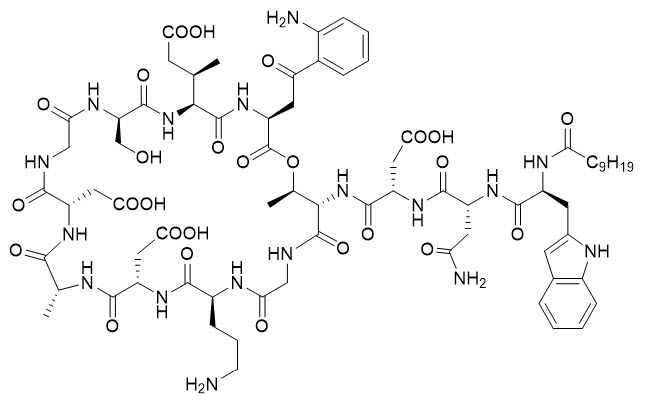
The empirical formula is C
6HOW SUPPLIED/STORAGE AND HANDLING
- DAPZURA RT (daptomycin for injection) is supplied as a sterile pale yellow to light brown lyophilized powder in a single-dose 10 mL vial containing 500 mg of daptomycin: Package of 1 (NDC 60977-145-01). The vial stopper is not made with natural rubber latex.
- Store original packages at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Storage conditions for the reconstituted and diluted solutions are described in another section of the prescribing information
7PATIENT COUNSELING INFORMATION
Allergic Reactions
Advise patients that allergic reactions, including serious skin, kidney, lung, or other organ reactions, could occur and that these serious reactions require immediate treatment. Patients should report any previous allergic reactions to daptomycin.
Muscle Pain or Weakness (Myopathy and Rhabdomyolysis, Peripheral Neuropathy)
Advise patients to report muscle pain or weakness, especially in the forearms and lower legs, as well as tingling or numbness.
Cough, Breathlessness or Fever (Eosinophilic Pneumonia)
Advise patients to report any symptoms of cough, breathlessness, or fever.
C. difficile-Associated Diarrhea (CDAD)
Advise patients that diarrhea is a common problem caused by antibacterials, including daptomycin for injection, that usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, including DAPZURA RT, patients can develop watery and bloody stools (with or without stomach cramps and fever), even as late as 2 or more months after having received the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible.
Patients with Hereditary Fructose Intolerance (HFI)
Inform patients and caregivers that DAPZURA RT contains sorbitol and can be life-threatening when administered to patients with hereditary fructose intolerance (HFI)
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including DAPZURA RT, should be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When DAPZURA RT is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be administered exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by DAPZURA RT or other antibacterial drugs in the future.
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in the USA
Baxter and Dapzura RT are trademarks of Baxter International Inc.
0719004045
8PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Barcode (FPO)
Use 10 mL
NDC 60977-
Store at 20°C to 25°C (68°F See package insert
for Usual Dosage and storage
of reconstituted and further
diluted product.
07-09-00-0427
for Usual Dosage and storage
of reconstituted and further
diluted product.
07-09-00-0427
Baxter Healthcare Corporation
DAPZURA RT
(daptomycin for injection)
For Intravenous Use
Single-dose vial –
Discard Unused Portion
Single-dose vial –
Discard Unused Portion
Baxter Logo
Lot: (FPO)
For Intravenous Use
Single-dose vial – Discard Unused Portion
Single-dose vial – Discard Unused Portion
Baxter Logo
2D Barcode Location
DAPZURA RT
(daptomycin for injection)
NDC 60977-
DAPZURA RT
(daptomycin for injection)
For Intravenous Use
Reconstitute viral only with
Single-dose vial -
1D Barcode
Recommended Dosage: See Prescribing
Information.
Information.
This package contains one single-dose
Not made with Natural rubber latex
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015
Deerfield, IL 60015
Made in the USA
2VR500
3-6118-520
NDC 60977-
DAPZURA RT
(daptomycin for injection)
For Intravenous Use
Reconstitute viral only with
Single-dose vial -
Rx Only
Baxter Logo
FPO 2D Barcode location
See enclosed package insert for
DAPZURA RT (daptomycin for injection)
Reconstitute with 10 mL Sterile Water
Each vial also contains 238 mg sorbitol
Contains no preservatives.
Note: Parenteral drug products should be