Brand Name
Dopram
Generic Name
Doxapram
View Brand Information FDA approval date: June 23, 1965
Classification: Respiratory Stimulant
Form: Injection
What is Dopram (Doxapram)?
Postanesthesia When the possibility of airway obstruction and/or hypoxia have been eliminated, doxapram may be used to stimulate respiration in patients with drug-induced postanesthesia respiratory depression or apnea other than that due to muscle relaxant drugs. To pharmacologically stimulate deep breathing in the postoperative patient. Drug-Induced Central Nervous System Depression Exercising care to prevent vomiting and aspiration, doxapram may be used to stimulate respiration, hasten arousal, and to encourage the return of laryngopharyngeal reflexes in patients with mild to moderate respiratory and CNS depression due to drug overdosage. Chronic Pulmonary Disease Associated with Acute Hypercapnia Doxapram is indicated as a temporary measure in hospitalized patients with acute respiratory insufficiency superimposed on chronic obstructive pulmonary disease. Its use should be for a short period of time as an aid in the prevention of elevation of arterial CO2 tension during the administration of oxygen. It should not be used in conjunction with mechanical ventilation.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
The Influence of Doxapram Administration on Diaphragmatic Excursion in Mechanically Ventilated Patients With Chronic Obstructive Pulmonary Disease Undergoing Spontaneous Breathing Trial: a Prospective Observational Study
Summary: Researchers aim to evaluate the impact of doxapram administration on diaphragmatic excursion in chronic obstructive pulmonary disease (COPD) patients undergoing spontaneous breathing trial
Doxapram Versus Placebo in Preterm Newborns: An International Double Blinded Multicenter Randomized Controlled Trial
Summary: Preterm infants often suffer from apnea of prematurity (AOP; a cessation of breathing) due to immaturity of the respiratory system. AOP can lead to oxygen shortage and a low heart rate which might harm the development of the newborn, especially the central nervous system. In order to prevent oxygen shortage, infants are treated with non-invasive respiratory support and caffeine. Despite these trea...
Related Latest Advances
Brand Information
Dopram (Doxapram hydrochloride)
1DESCRIPTION
DOPRAM Injection (doxapram hydrochloride injection, USP) is a clear, colorless, sterile, non-pyrogenic, aqueous solution with pH 3.5 to 5, for intravenous administration.
Each 1 mL contains:
Doxapram Hydrochloride, USP ................................................................. 20 mg
Doxapram Injection is a respiratory stimulant.
Doxapram hydrochloride is a white to off-white, crystalline powder, sparingly soluble in water, alcohol and chloroform. Chemically, doxapram hydrochloride is 1-ethyl-4-[2-(4-morpholinyl)ethyl]-3,3-diphenyl-2-pyrrolidinone monohydrochloride, monohydrate.
The chemical structure is:
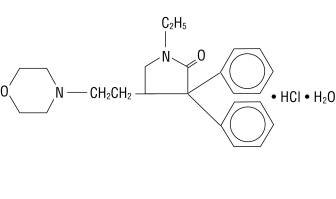
C
2CONTRAINDICATIONS
Doxapram is contraindicated in patients with known hypersensitivity to the drug or any of the injection components.
Doxapram should not be used in patients with epilepsy or other convulsive disorders.
Doxapram is contraindicated in patients with proven or suspected pulmonary embolism.
Doxapram is contraindicated in patients with mechanical disorders of ventilation such as mechanical obstruction, muscle paresis (including neuromuscular blockade), flail chest, pneumothorax, acute bronchial asthma, pulmonary fibrosis, or other conditions resulting in restriction of the chest wall, muscles of respiration, or alveolar expansion.
Doxapram is contraindicated in patients with evidence of head injury, cerebral vascular accident, or cerebral edema, and in those with significant cardiovascular impairment, uncompensated heart failure, severe coronary artery disease, or severe hypertension, including that associated with hyperthyroidism or pheochromocytoma. (See
3WARNINGS
Doxapram should not be used in conjunction with mechanical ventilation.
Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications containing this preservative must take into account the total amount of benzyl alcohol administered. The amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources (see
3.1In Postanesthetic Use
- Doxapram is neither an antagonist to muscle relaxant drugs nor a specific narcotic antagonist. More specific tests (eg, peripheral nerve stimulation, airway pressures, head lift, pulse oximetry, and end-tidal carbon dioxide) to assess adequacy of ventilation are recommended before administering doxapram.
- Doxapram should be administered with great care and only under careful supervision to patients with hypermetabolic states such as hyperthyroidism or pheochromocytoma.
- Since narcosis may recur after stimulation with doxapram, care should be taken to maintain close observation until the patient has been fully alert for ½ to 1 hour.
- In patients who have received general anesthesia utilizing a volatile agent known to sensitize the myocardium to catecholamines, administration of doxapram should be delayed until the volatile agent has been excreted in order to lessen the potential for arrhythmias, including ventricular tachycardia and ventricular fibrillation (see
3.2In Drug-Induced CNS and Respiratory Depression
Doxapram alone may not stimulate adequate spontaneous breathing or provide sufficient arousal in patients who are
3.3In Chronic Obstructive Pulmonary Disease
Because of the associated increased work of breathing, do not increase the rate of infusion of doxapram in severely ill patients in an attempt to lower pCO
4ADVERSE REACTIONS
Adverse reactions reported coincident with the administration of DOPRAM (doxapram hydrochloride, USP) include:
4.1Central and Autonomic Nervous Systems
Pyrexia, flushing, sweating; pruritus and paresthesia, such as a feeling of warmth, burning, or hot sensation, especially in the area of genitalia and perineum; apprehension, disorientation, pupillary dilatation, hallucinations, headache, dizziness, hyperactivity, involuntary movements, muscle spasticity, muscle fasciculations, increased deep tendon reflexes, clonus, bilateral Babinski, and convulsions.
4.2Respiratory
Dyspnea, cough, hyperventilation, tachypnea, laryngospasm, bronchospasm, hiccough, and rebound hypoventilation.
4.3Cardiovascular
Phlebitis, variations in heart rate, lowered T-waves, arrhythmias (including ventricular tachycardia and ventricular fibrillation), chest pain, tightness in chest. A mild to moderate increase in blood pressure is commonly noted and may be of concern in patients with severe cardiovascular diseases.
4.4Gastrointestinal
Nausea, vomiting, diarrhea, desire to defecate.
4.5Genitourinary
Stimulation of urinary bladder with spontaneous voiding; urinary retention. Elevation of BUN and albuminuria.
4.6Hemic and Lymphatic
Hemolysis with rapid infusion. A decrease in hemoglobin, hematocrit, or red blood cell count has been observed in postoperative patients. In the presence of pre-existing leukopenia, a further decrease in WBC has been observed following anesthesia and treatment with doxapram hydrochloride.
5DOSAGE AND ADMINISTRATION
5.1In the Management of Drug-Induced CNS Depression
(See Table II. Dosage for drug-induced CNS depression.)
5.2Chronic Obstructive Pulmonary Disease Associated with Acute Hypercapnia
- One vial of doxapram (400 mg) should be mixed with 180 mL of dextrose 5% or 10% or normal saline solution (concentration of 2 mg/mL). The infusion should be started at 1 to 2 mg/minute (½ to 1 mL/minute); if indicated, increase to a maximum of 3 mg/minute. Arterial blood gases should be determined prior to the onset of doxapram’s administration and at least every half hour during the two hours of infusion to insure against the insidious development of CO
- Predictable blood gas patterns are more readily established with a continuous infusion of doxapram. If the blood gases show evidence of deterioration, the infusion of doxapram should be discontinued.
- ADDITIONAL INFUSIONS BEYOND THE SINGLE MAXIMUM TWO HOUR ADMINISTRATION PERIOD ARE NOT RECOMMENDED.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
5.3Diluent Compatibility
Doxapram hydrochloride is compatible with 5% and 10% dextrose in water or normal saline.
5.4Incompatibility
ADMIXTURE OF DOXAPRAM WITH ALKALINE SOLUTIONS SUCH AS 2.5% THIOPENTAL SODIUM, SODIUM BICARBONATE, FUROSEMIDE, OR AMINOPHYLLINE WILL RESULT IN PRECIPITATION OR GAS FORMATION.
Doxapram is also not compatible with ascorbic acid, cefoperazone sodium, cefotaxime sodium, cefotetan sodium, cefuroxime sodium, folic acid, dexamethasone disodium phosphate, diazepam, hydrocortisone sodium phosphate, methylprednisolone sodium, or hydrocortisone sodium succinate.
Admixture of doxapram and ticarcillin disodium results in an 18% loss of doxapram in 3 hours. When doxapram is mixed with minocycline hydrochloride, there is a loss of 8% of doxapram in 3 hours and a 13% loss of doxapram in 6 hours.
6HOW SUPPLIED
DOPRAM Injection (doxapram hydrochloride injection, USP) is available in cartons of one 20 mL multiple dose vial containing 20 mg of doxapram hydrochloride per mL with benzyl alcohol 0.9% as the preservative (NDC 0641-6018-01).
Store at Controlled Room Temperature, Between 20˚C to 25˚C (68˚F to 77˚F). See USP.
7PACKAGE LABELING - PRINCIPAL DISPLAY PANEL
NDC 0641-


