Generic Name
Sofosbuvir
Brand Names
Sovaldi, Vosevi, Epclusa, Ledipasvir, Harvoni
FDA approval date: December 06, 2013
Classification: Hepatitis C Virus NS5A Inhibitor
Form: Pellet, Tablet
What is Sovaldi (Sofosbuvir)?
HARVONI is indicated for the treatment of adults and pediatric patients 3 years of age and older with chronic hepatitis C virus [see Dosage and Administration.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Sovaldi (SOFOSBUVIR)
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with SOVALDI. HBV reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals and were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Monitor HCV/HBV coinfected patients for hepatitis flare or HBV reactivation during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated
1DOSAGE FORMS AND STRENGTHS
SOVALDI is available as tablets or pellets for oral use. Each dosage form is available in two dose strengths.
- 400 mg Tablets: 400 mg sofosbuvir: yellow, capsule-shaped, film-coated tablet debossed with "GSI" on one side and "7977" on the other side.
- 200 mg Tablets: 200 mg sofosbuvir: yellow, oval-shaped, film-coated tablet debossed with "GSI" on one side and "200" on the other side.
- 200 mg Pellets: 200 mg sofosbuvir: white to off-white pellets in unit-dose packets.
- 150 mg Pellets: 150 mg sofosbuvir: white to off-white pellets in unit-dose packets.
2CONTRAINDICATIONS
When SOVALDI is used in combination with ribavirin or peginterferon alfa/ribavirin, the contraindications applicable to those agents are applicable to combination therapies. Refer to the prescribing information of peginterferon alfa and ribavirin for a list of their contraindications.
3ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Serious Symptomatic Bradycardia When Coadministered with Amiodarone
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
When SOVALDI is administered with ribavirin or peginterferon alfa/ribavirin, refer to the respective prescribing information for a description of adverse reactions associated with their use.
3.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of SOVALDI. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
4OVERDOSAGE
The highest documented dosage of sofosbuvir was a single dose of sofosbuvir 1200 mg (three times the recommended dosage) administered to 59 healthy subjects. In that trial, there were no untoward effects observed at this dosage level, and adverse events were similar in frequency and severity to those reported in the placebo and sofosbuvir 400 mg treatment groups. The effects of higher dosages are not known.
No specific antidote is available for overdose with SOVALDI. If overdose occurs, the patient must be monitored for evidence of toxicity. Treatment of overdose with SOVALDI consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. A 4-hour hemodialysis session removed 18% of the administered dose.
5DESCRIPTION
SOVALDI (sofosbuvir) is a nucleotide analog inhibitor of HCV NS5B polymerase.
The IUPAC name for sofosbuvir is (
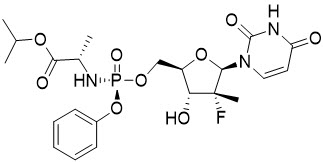
Sofosbuvir is a white to off-white crystalline solid with a solubility of ≥ 2 mg/mL across the pH range of 2–7.7 at 37 °C and is slightly soluble in water.
SOVALDI tablets, 200 mg or 400 mg, are for oral administration. Each tablet contains 200 mg or 400 mg of sofosbuvir. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose. The tablets are film-coated with a coating material containing the following inactive ingredients: polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
SOVALDI pellets, 150 mg or 200 mg, are for oral administration, supplied as white to off-white pellets in unit-dose packets. Each unit-dose packet contains 150 mg or 200 mg of sofosbuvir. The pellets include the following inactive ingredients: amino methacrylate copolymer, colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, sodium lauryl sulfate, sodium stearyl fumarate, stearic acid, and talc.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
7INSTRUCTIONS FOR USE
SOVALDI
Read the Patient Information that comes with SOVALDI oral pellets for important information about SOVALDI.
This Instructions for Use contains information on how to take SOVALDI oral pellets. Be sure you understand and follow the instructions. If you have any questions, ask your healthcare provider or pharmacist.
Important Information You Need to Know Before Taking SOVALDI oral pellets
- For oral use only (take by mouth with or without food).
- Do not open the SOVALDI oral pellet packet(s) until ready to use.
- SOVALDI oral pellets are white to off-white pellets supplied as single-use packets in cartons. Each carton contains 28 packets.
- Do not use SOVALDI oral pellets if the carton tamper-evident seal, or the pellets packet seal, is broken or damaged.
Before you prepare a dose of SOVALDI oral pellets to be taken with food, gather the following supplies:
- Daily SOVALDI oral pellet packet(s), as prescribed by your healthcare provider
- One or more spoonfuls of non-acidic soft food such as pudding, chocolate syrup, mashed potato, or ice cream
- Bowl
- Spoon
- Scissors (optional)
Step 1: Add one or more spoonfuls of non-acidic soft food to the bowl first.
Step 4: Cut the packet along the cut line with scissors (see Figure C), or fold the packet back at the tear line (see Figure D) and tear open (see Figure E).
Step 5: Carefully pour the entire contents of the prescribed number of SOVALDI oral pellet packet(s) onto the food in the bowl and gently mix with a spoon (see Figure F). Make sure that no SOVALDI oral pellets remain in the packet(s).
Step 6: Take the SOVALDI oral pellets and food mixture within 30 minutes without chewing to avoid a bitter taste. Ensure all of the SOVALDI oral pellets are taken.
Before you prepare a dose of SOVALDI oral pellets to be taken without food, gather the following supplies:
- Daily SOVALDI oral pellet packet(s), as prescribed by your healthcare provider
- Scissors (optional)
- Water (optional)
Step 3: Cut the packet along the cut line with scissors (see Figure I), or fold the packet back at the tear line (see Figure J) and tear open (see Figure K).
Step 4: Pour the entire contents of the SOVALDI oral pellets packet directly in the mouth and swallow without chewing to avoid a bitter taste (see Figure L). Water may be taken after swallowing the pellets, if needed. Make sure that no SOVALDI oral pellets remain in the packet. If your healthcare provider prescribed more than one SOVALDI oral pellets packet, repeat Steps 1 through 4.
Storing SOVALDI oral pellets
- Store SOVALDI pellets below 86°F (30°C).
Disposing of SOVALDI oral pellets
- Throw away any unused portion. Do not store and reuse any leftover SOVALDI mixture (pellets mixed with food).
For more information, call 1-800-445-3235 or go to www.SOVALDI.com.
Manufactured for and distributed by: Gilead Sciences, Inc., Foster City, CA 94404
© 2024 Gilead Sciences, Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
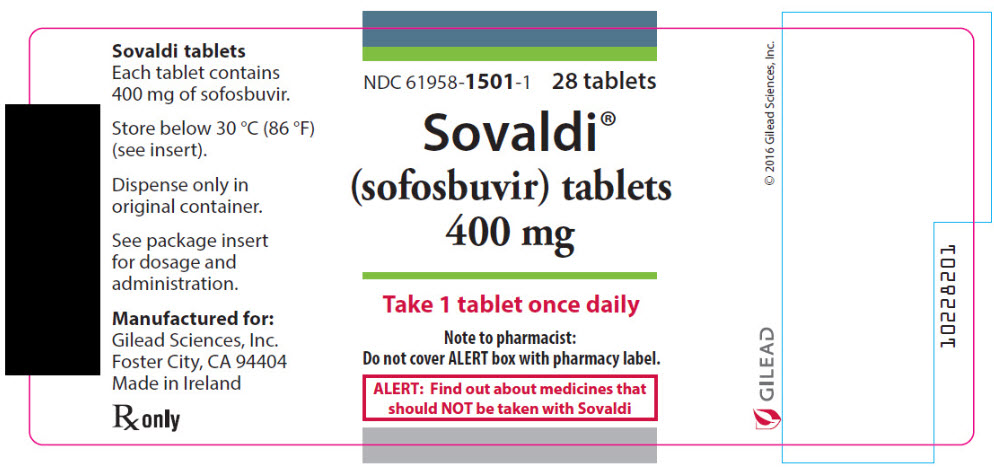
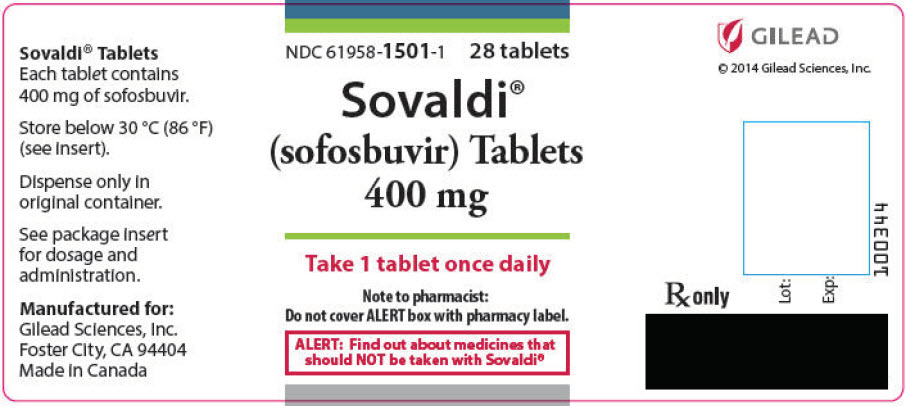
8PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label
NDC 61958-1501-1
Sovaldi
Take 1 tablet once daily
Note to pharmacist:
ALERT: Find out about medicines that
9PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
NDC 61958-1503-1
Sovaldi
200 mg
Take 1 tablet once daily
Note to pharmacist:
ALERT: Find out about medicines that
10PRINCIPAL DISPLAY PANEL - 150 mg Pellet Packet Carton Label
NDC 61958-
Sovaldi
150 mg per packet
Rx only
Note to pharmacist: Do not cover ALERT box with pharmacy label.
ALERT: Find out about medicines that should NOT be taken with Sovaldi
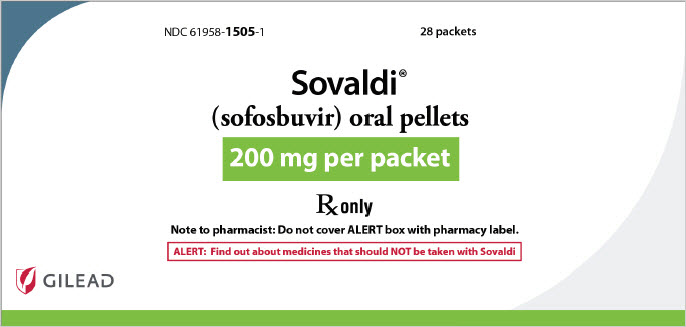
11PRINCIPAL DISPLAY PANEL - 200 mg Pellet Packet Carton Label
NDC 61958-
Sovaldi
200 mg per packet
Rx only
Note to pharmacist: Do not cover ALERT box with pharmacy label.
ALERT: Find out about medicines that should NOT be taken with Sovaldi