Brand Name
Zarontin
Generic Name
Ethosuximide
View Brand Information FDA approval date: September 22, 2000
Classification: Anti-epileptic Agent
Form: Capsule, Solution
What is Zarontin (Ethosuximide)?
Ethosuximide capsule is indicated for the control of absence epilepsy.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Comparative Study to Assess Safety and Effectiveness of Ethosuximide and Pentoxifylline in the Treatment of Abdominal Pain Related to Irritable Bowel Syndrome
Summary: Ethosuximide and pentoxifylline in the treatment of abdominal pain related to irritable bowel syndrome
Related Latest Advances
Brand Information
Zarontin (Ethosuximide)
1DESCRIPTION
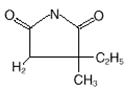
Zarontin (ethosuximide) is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula:
Each Zarontin capsule contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol 400, NF. The capsule contains D&C yellow No. 10; FD&C red No. 3; gelatin, NF; glycerin, USP; and sorbitol.
2CLINICAL PHARMACOLOGY
Ethosuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence (petit mal) seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli.
3INDICATIONS AND USAGE
Zarontin is indicated for the control of absence (petit mal) epilepsy.
4CONTRAINDICATION
Ethosuximide should not be used in patients with a history of hypersensitivity to succinimides.
5ADVERSE REACTIONS
Body As A Whole: Allergic reaction, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
Gastrointestinal System: Gastrointestinal symptoms occur frequently and include anorexia, vague gastric upset, nausea and vomiting, cramps, epigastric and abdominal pain, weight loss, and diarrhea. There have been reports of gum hypertrophy and swelling of the tongue.
Hemopoietic System: Hemopoietic complications associated with the administration of ethosuximide have included leukopenia, agranulocytosis, pancytopenia, with or without bone marrow suppression, eosinophilia, and thrombocytopenia (see WARNINGS).
Nervous System: Neurologic and sensory reactions reported during therapy with ethosuximide have included drowsiness, headache, dizziness, euphoria, hiccups, irritability, hyperactivity, lethargy, fatigue, and ataxia. Psychiatric or psychological aberrations associated with ethosuximide administration have included disturbances of sleep, night terrors, inability to concentrate, and aggressiveness. These effects may be noted particularly in patients who have previously exhibited psychological abnormalities. There have been rare reports of paranoid psychosis, increased libido, and increased state of depression with overt suicidal intentions.
Integumentary System: Dermatologic manifestations which have occurred with the administration of ethosuximide have included urticaria, pruritic erythematous rashes, Stevens-Johnson syndrome, and hirsutism.
Special Senses: Myopia.
Genitourinary System: Vaginal bleeding, microscopic hematuria.
6OVERDOSAGE
Acute overdoses may produce nausea, vomiting, and CNS depression including coma with respiratory depression. A relationship between ethosuximide toxicity and its plasma levels has not been established. The therapeutic range of serum levels is 40 mcg/mL to 100 mcg/mL, although levels as high as 150 mcg/mL have been reported without signs of toxicity.
6.1Treatment:
Treatment should include emesis (unless the patient is or could rapidly become obtunded, comatose, or convulsing) or gastric lavage, activated charcoal, cathartics, and general supportive measures. Hemodialysis may be useful to treat ethosuximide overdose. Forced diuresis and exchange transfusions are ineffective.
7DOSAGE AND ADMINISTRATION
Zarontin is administered by the oral route. The
Zarontin may be administered in combination with other anticonvulsants when other forms of epilepsy coexist with absence (petit mal). The
8HOW SUPPLIED
Zarontin is supplied as:
NDC 0071-0237-24: Bottles of 100. Each capsule contains 250 mg ethosuximide.
9MEDICATION GUIDE ZARONTIN, (Ză rŏn' tĭn) (ethosuximide) Capsules, Oral Solution
Read this Medication Guide before you start taking ZARONTIN and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about ZARONTIN, ask your healthcare provider or pharmacist.
What is the most important information I should know about ZARONTIN?
Do not stop taking ZARONTIN without first talking to your healthcare provider.
Stopping ZARONTIN suddenly can cause serious problems.
Stopping ZARONTIN suddenly can cause serious problems.
ZARONTIN can cause serious side effects, including:
- Rare but serious blood problems that may be life-threatening. Call your healthcare provider right away if you have:
- fever, swollen glands, or sore throat that come and go or do not go away
- frequent infections or an infection that does not go away
- easy bruising
- red or purple spots on your body
- bleeding gums or nose bleeds
- severe fatigue or weakness
- Drug reactions that may affect different parts of the body such as your liver, kidneys, heart, or blood cells. You may or may not have a rash with these types of reactions. These reactions can be very serious and can cause death. Call your healthcare provider right away if you have any of these symptoms:
- joint pain and swelling
- muscle pain
- fatigue, weakness
- low-grade fever
- pain in the chest that is worse with breathing
- skin rash
- swollen lymph glands
- swelling of your face
- yellowing of your skin or the white part of your eyes
- Like other antiepileptic drugs, ZARONTIN may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
- Do not stop ZARONTIN without first talking to a healthcare provider.
- Stopping ZARONTIN suddenly can cause serious problems.
- Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
What is ZARONTIN?
ZARONTIN is a prescription medicine used to treat absence (petit mal) seizures.
Who should not take ZARONTIN?
Do not take ZARONTIN if you are allergic to succinimides (methsuximide or ethosuximide), or any of the ingredients in ZARONTIN. See the end of this Medication Guide for a complete list of ingredients in ZARONTIN.
What should I tell my healthcare provider before taking ZARONTIN?
Before you take ZARONTIN, tell your healthcare provider if you:
- have or had liver problems
- have or have had depression, mood problems or suicidal thoughts or behavior
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if ZARONTIN can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking ZARONTIN. You and your healthcare provider should decide if you should take ZARONTIN while you are pregnant.
- are breast-feeding or plan to breast-feed. It is not known if ZARONTIN can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take ZARONTIN.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Taking ZARONTIN with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ZARONTIN?
- Take ZARONTIN exactly as prescribed. Your healthcare provider will tell you how much ZARONTIN to take.
- Your healthcare provider may change your dose. Do not change your dose of ZARONTIN without talking to your healthcare provider.
- If you take too much ZARONTIN, call your healthcare provider or your local Poison Control Center right away.
What should I avoid while taking ZARONTIN?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking ZARONTIN without first talking to your healthcare provider. ZARONTIN taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
What are the possible side effects of ZARONTIN?
- See "What is the most important information I should know about ZARONTIN?"
ZARONTIN may cause other serious side effects, including:
- Serious allergic reactions. Call your healthcare provider right away if you have any of these symptoms:
- Changes in thinking, mood, or behavior. Some patients may get abnormally suspicious thoughts, hallucinations (seeing or hearing things that are not there), or delusions (false thoughts or beliefs).
- Grand mal seizures can happen more often or become worse
Call your healthcare provider right away, if you have any of the symptoms listed above.
The most common side effects of ZARONTIN include
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects with ZARONTIN. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
How should I store ZARONTIN?
- Store ZARONTIN capsules at room temperature, between 59°F to 86°F (15°C to 30°C).
- Store ZARONTIN syrup (oral solution) at 20°–25°C (68°–77°F). Preserve in tight containers. Protect from freezing and light.
Keep ZARONTIN and all medicines out of the reach of children.
General information about ZARONTIN
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZARONTIN for a condition for which it was not prescribed. Do not give ZARONTIN to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about ZARONTIN. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about ZARONTIN that is written for healthcare professionals.
For more information, go to www.pfizer.com or call 1-800-438-1985.
What are the ingredients in ZARONTIN?
Active ingredient: ethosuximide
Capsules
Inactive ingredients: Polyethylene glycol 400, NF; D&C yellow No. 10; FD&C red No. 3; gelatin, NF; glycerin, USP; and sorbitol.
Inactive ingredients: Polyethylene glycol 400, NF; D&C yellow No. 10; FD&C red No. 3; gelatin, NF; glycerin, USP; and sorbitol.
Oral Solution
Inactive ingredients: Each 5 ml (teaspoonful) of oral solution contains 250 mg ethosuximide in a raspberry flavored base. Also contains citric acid, anhydrous, USP; FD&C red No. 40; FD&C yellow No. 6; flavor; glycerin, USP; purified water, USP; saccharin sodium, USP; sodium benzoate, NF; Sodium Citrate, USP; sucrose, NF.
Inactive ingredients: Each 5 ml (teaspoonful) of oral solution contains 250 mg ethosuximide in a raspberry flavored base. Also contains citric acid, anhydrous, USP; FD&C red No. 40; FD&C yellow No. 6; flavor; glycerin, USP; purified water, USP; saccharin sodium, USP; sodium benzoate, NF; Sodium Citrate, USP; sucrose, NF.
This Medication Guide has been approved by the U.S. Food and Drug Administration.

LAB-0403-4.0
June 2023
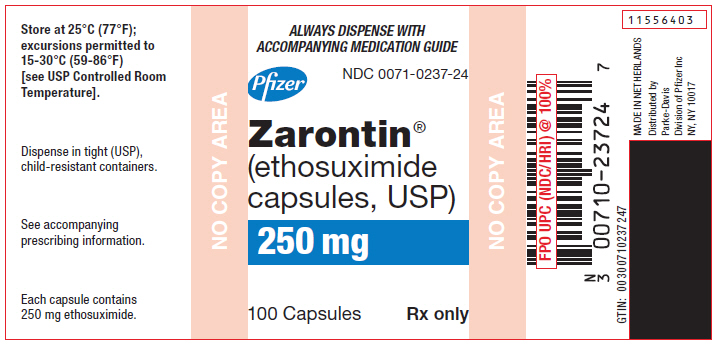
10PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle Label
ALWAYS DISPENSE WITH
ACCOMPANYING MEDICATION GUIDE
ACCOMPANYING MEDICATION GUIDE
NDC 0071-0237-24
Pfizer
Zarontin
(ethosuximide
capsules, USP)
(ethosuximide
capsules, USP)
250 mg
100 Capsules