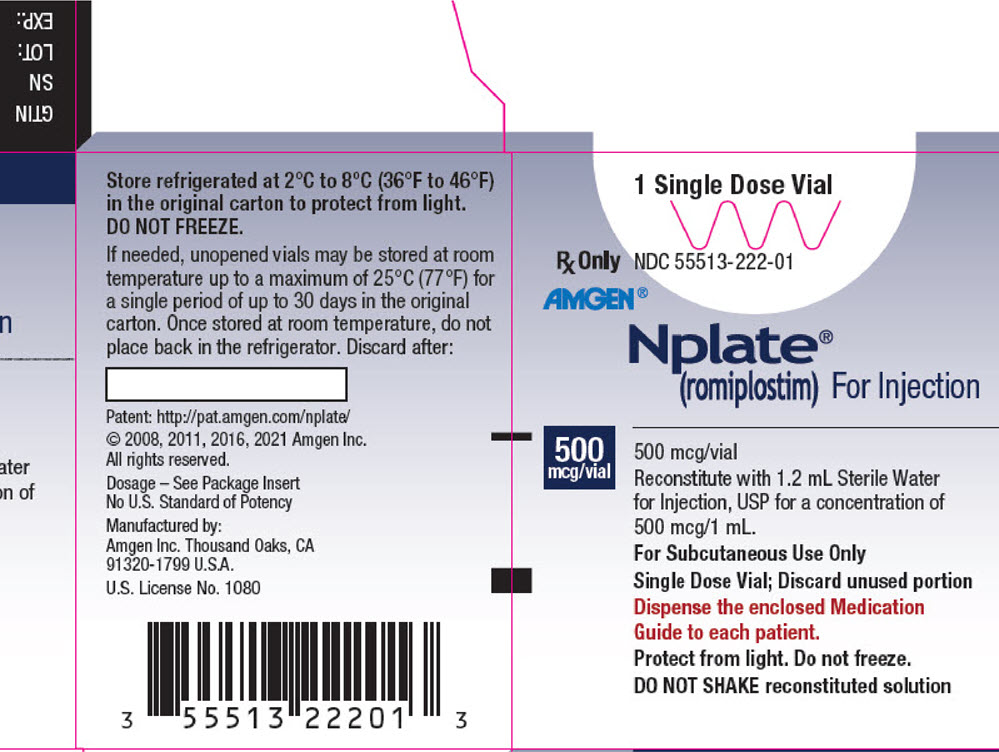
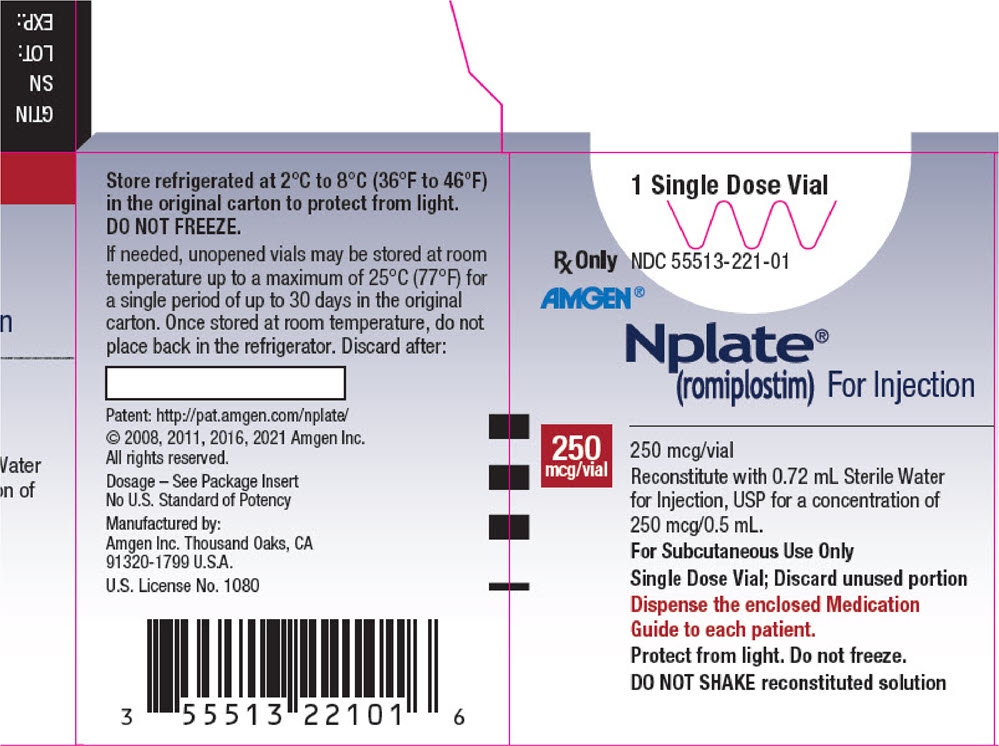
Nplate
What is Nplate (Romiplostim)?
Approved To Treat
Related Clinical Trials
Summary: This is a prospective, randomized, controlled clinical study designed to evaluate the efficacy and safety of Romiplostim N01 in promoting platelet engraftment after haploidentical allogeneic hematopoietic stem cell transplantation (haplo-HSCT) in patients with hematologic malignancies. A total of 130 patients who undergo haplo-HSCT for acute myeloid leukemia (AML), myelodysplastic syndromes (MDS),...
Summary: The goal of this clinical trial is to to assess the efficacy of romiplostim as a supportive care measure in patients with a new diagnosis of Ewing sarcoma receiving interval-compressed chemotherapy. The main questions it aims to answer are: 1. To demonstrate the efficacy of romiplostim in patients with newly diagnosed Ewing sarcoma, measured specifically as the rate of chemotherapy-induced thrombo...
Summary: The primary objective of this trial is to assess the efficacy and safety of combining Romiplostim N01 with Rituximab for the treatment of adult patients with primary immune thrombocytopenia (ITP) whose disease is refractory to oral TPO-RAs. All participants in this study will receive the same combination treatment: Rituximab: Given once a week through an intravenous infusion for 4 weeks. Romiplost...
Related Latest Advances
Brand Information
- Progression of Myelodysplastic Syndromes
- Thrombotic/Thromboembolic Complications
- Loss of Response to Nplate
- Erythromelalgia
- Hypersensitivity reactions including angioedema and anaphylaxis
- Nplate therapy is administered to achieve and maintain a platelet count ≥ 50 × 10
- Following discontinuation of Nplate, thrombocytopenia and risk of bleeding may develop that is worse than that experienced prior to the Nplate therapy.
- Nplate therapy may increase the risk of reticulin fiber formation within the bone marrow. This formation may improve upon discontinuation. Detection of peripheral blood cell abnormalities may necessitate a bone marrow examination.
- Too much Nplate may result in excessive platelet counts and a risk for thrombotic/thromboembolic complications.
- Nplate stimulates certain bone marrow cells to make platelets and increases the risk of progression to acute myelogenous leukemia in patients with myelodysplastic syndromes.
- Platelet counts and CBCs must be performed weekly until a stable Nplate dose has been achieved; thereafter, platelet counts and CBCs must be performed monthly while taking Nplate.
- Patients must be closely monitored with weekly platelet counts and CBCs for at least 2 weeks following Nplate discontinuation.
- Even with Nplate therapy, patients should continue to avoid situations or medications that may increase the risk for bleeding.
- Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy
- Advise women not to breastfeed during treatment with Nplate
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
U.S. License No. 1080