Generic Name
Clozapine
Brand Names
Clozaril, Versacloz
FDA approval date: July 08, 1999
Classification: Atypical Antipsychotic
Form: Tablet, Suspension
What is Clozaril (Clozapine)?
Clozapine is an atypical antipsychotic indicated for: Treatment-resistant schizophrenia. Efficacy was established in an active-controlled study.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Clozaril (clozapine)
WARNING: SEVERE NEUTROPENIA; ORTHOSTATIC HYPOTENSION, BRADYCARDIA, AND SYNCOPE; SEIZURE; MYOCARDITIS, PERICARDITIS AND CARDIOMYOPATHY; INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Severe Neutropenia
CLOZARIL has caused severe neutropenia which is associated with an increased risk of serious and potentially fatal infections. Prior to initiating CLOZARIL treatment, obtain baseline ANC(s). CLOZARIL initiation is not recommended in patients with a baseline ANC less than 1500/μL (less than 1000/μL for those with Benign Ethnic Neutropenia (also known as Duffy-null associated neutrophil count)). See recommendations for dosage modifications based on ANC levels during CLOZARIL treatment .
Orthostatic Hypotension, Bradycardia, Syncope
Orthostatic hypotension, bradycardia, syncope, and cardiac arrest have occurred with CLOZARIL treatment. The risk is highest during the initial titration period, particularly with rapid dose escalation. These reactions can occur with the first dose, with doses as low as 12.5 mg per day, or when restarting patients who have had even a brief interruption in treatment with CLOZARIL. Initiate treatment at 12.5 mg once or twice daily; titrate slowly; and use divided dosages to minimize risk. Use CLOZARIL cautiously in patients with cardiovascular or cerebrovascular disease or conditions predisposing to hypotension (e.g., dehydration, use of antihypertensive medications)
Seizures
Seizures have occurred with CLOZARIL treatment. The risk is dose-related. Initiate treatment at 12.5 mg, titrate gradually, and use divided dosing. Use caution when administering CLOZARIL to patients with a history of seizures or other predisposing risk factors for seizure (CNS pathology, medications that lower the seizure threshold, alcohol abuse). Caution patients about engaging in any activity where sudden loss of consciousness could cause serious risk to themselves or others
Myocarditis, Pericarditis, Cardiomyopathy and Mitral Valve Incompetence
Fatal myocarditis and cardiomyopathy have occurred with CLOZARIL treatment. Discontinue CLOZARIL and obtain a cardiac evaluation upon suspicion of these reactions. Generally, patients with CLOZARIL-related myocarditis or cardiomyopathy should not be rechallenged with CLOZARIL. Consider the possibility of myocarditis, pericarditis, or cardiomyopathy if chest pain, tachycardia, palpitations, dyspnea, fever, flu-like symptoms, hypotension, or ECG changes occur [see
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. CLOZARIL is not approved for use in patients with dementia-related psychosis
1DOSAGE FORMS AND STRENGTHS
Tablets: 25 mg and 100 mg round, pale-yellow, with a facilitated score on one side.
2CONTRAINDICATIONS
CLOZARIL is contraindicated in patients with a history of hypersensitivity to clozapine (e.g., photosensitivity, vasculitis, erythema multiforme, or Stevens-Johnson syndrome) or any other component of CLOZARIL 6.2)].
3ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Severe Neutropenia
- Orthostatic Hypotension, Bradycardia, and Syncope
- Falls
- Seizures
- Myocarditis, Pericarditis, Cardiomyopathy, and Mitral Valve Incompetence
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Gastrointestinal Hypomotility with Severe Complications
- Eosinophilia
- QT Interval Prolongation
- Metabolic Changes (Hyperglycemia and Diabetes Mellitus, Dyslipidemia, and Weight Gain)
- Neuroleptic Malignant Syndrome
- Hepatotoxicity
- Fever
- Pulmonary Embolism
- Anticholinergic Toxicity
- Interference with Cognitive and Motor Performance
- Tardive Dyskinesia
- Cerebrovascular Adverse Reactions
- Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most commonly reported adverse reactions (≥5%) across CLOZARIL clinical trials were: CNS reactions, including sedation, dizziness/vertigo, headache, and tremor; cardiovascular reactions, including tachycardia, hypotension, and syncope; autonomic nervous system reactions, including hypersalivation, sweating, dry mouth, and visual disturbances; gastrointestinal reactions, including constipation and nausea; and fever.
Table 10 summarizes the adverse reactions reported in CLOZARIL-treated patients at a frequency of 2% or greater across all CLOZARIL studies (excluding the 2-year InterSePT™ Study). These rates are not adjusted for duration of exposure.
Table 11 summarizes the most commonly reported adverse reactions (≥10% of the CLOZARIL or olanzapine group) in the InterSePT™ Study. This was an adequate and well-controlled, two-year study evaluating the efficacy of CLOZARIL relative to olanzapine in reducing the risk of suicidal behavior in patients with schizophrenia or schizoaffective disorder. The rates are not adjusted for duration of exposure.
Dystonia
Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of clozapine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Central Nervous System
Delirium, EEG abnormal, myoclonus, paresthesia, possible cataplexy, status epilepticus, obsessive compulsive symptoms, restless leg syndrome and post-discontinuation cholinergic rebound adverse reactions.
Cardiovascular System
Atrial or ventricular fibrillation, ventricular tachycardia, palpitations, QT interval prolongation, Torsades de Pointes, mitral valve incompetence associated with clozapine-related cardiomyopathy, myocardial infarction, cardiac arrest, myocarditis, pericarditis and periorbital edema.
Endocrine System
Pseudopheochromocytoma
Gastrointestinal System
Acute pancreatitis, dysphagia, salivary gland swelling, colitis, megacolon, fecal incontinence, and intestinal ischemia, infarction, perforation, ulceration or necrosis.
Hepatobiliary System
Cholestasis, hepatitis, jaundice, hepatotoxicity, hepatic steatosis, hepatic necrosis, hepatic fibrosis, hepatic cirrhosis, liver injury (hepatic, cholestatic, and mixed), and liver failure.
Immune System Disorders
Angioedema, leukocytoclastic vasculitis.
Urogenital System
Acute interstitial nephritis, nocturnal enuresis, priapism, renal failure, and retrograde ejaculation.
Skin and Subcutaneous Tissue Disorders
Hypersensitivity reactions: photosensitivity, vasculitis, erythema multiforme, skin pigmentation disorder, and Stevens-Johnson Syndrome.
Musculoskeletal System and Connective Tissue Disorders
Myasthenic syndrome, rhabdomyolysis, and systemic lupus erythematosus.
Respiratory System
Aspiration, pleural effusion, pneumonia, lower respiratory tract infection, sleep apnea.
Hemic and Lymphatic System
Mild, moderate, or severe leukopenia, agranulocytosis, granulocytopenia, WBC decreased, deep-vein thrombosis, elevated hemoglobin/hematocrit, erythrocyte sedimentation rate (ESR) increased, sepsis, thrombocytosis, and thrombocytopenia.
Vision Disorders
Narrow-angle glaucoma.
Miscellaneous
Creatine phosphokinase elevation, hyperuricemia, hyponatremia, polyserositis, and weight loss.
4DESCRIPTION
CLOZARIL
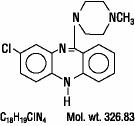
CLOZARIL is available in pale yellow tablets of 25 mg and 100 mg for oral administration.
Active Ingredient: clozapine
Inactive Ingredients are colloidal silicon dioxide, lactose, magnesium stearate, povidone, starch (corn), and talc.
5PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). Discuss the following issues with patients and caregivers:
- Severe Neutropenia:
Instruct patients (and caregivers)
- About the risk of developing severe neutropenia and infection with CLOZARIL treatment.
- Instruct patients to immediately report to their health care provider any symptom or sign of during CLOZARIL treatment.
- About the importance of having frequent ANC testing.
- Orthostatic Hypotension, Bradycardia, and Syncope: Inform patients and caregivers about the risk of orthostatic hypotension and syncope, especially during the period of initial dose titration. Instruct them to strictly follow the clinician’s instructions for dosage and administration [see Dosage and Administration (2.6)]. Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of bradycardia or arrhythmia [Warnings and Precautions (.
- Seizures: Inform patients and caregivers about the significant risk of seizure during CLOZARIL treatment. Caution them about driving and any other potentially hazardous activity while taking CLOZARIL [see Warnings and Precautions (.
- Gastrointestinal Hypomotility with Severe Complications: Educate patients and caregivers on the risks, prevention and treatment of clozapine-induced constipation, including medications to avoid when possible (e.g., drugs with anticholinergic activity). Encourage appropriate hydration, physical activity, and fiber intake and emphasize that prompt attention and treatment to the development of constipation or other gastrointestinal symptoms is critical in preventing severe complications. Advise patients and caregivers to contact their health care provider if they experience symptoms of constipation (e.g., difficulty passing stools, incomplete passage of stool, decreased bowel movement frequency) or other symptoms associated with gastrointestinal hypomotility (e.g., nausea, abdominal distension or pain, vomiting) [see Warnings and Precautions (.
- QT Interval Prolongation: Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of arrhythmia. Instruct patients to not take CLOZARIL with other drugs that cause QT interval prolongation. Instruct patients to inform their clinicians that they are taking CLOZARIL before any new drug [see Warnings and Precautions (.
- Metabolic Changes (hyperglycemia and diabetes mellitus, dyslipidemia, weight gain): Educate patients and caregivers about the risk of metabolic changes and the need for specific monitoring. The risks include hyperglycemia and diabetes mellitus, dyslipidemia, weight gain, and cardiovascular reactions. Educate patients and caregivers about the symptoms of hyperglycemia (high blood sugar) and diabetes mellitus (e.g., polydipsia, polyuria, polyphagia, and weakness). Monitor all patients for these symptoms. Patients who are diagnosed with diabetes or have risk factors for diabetes (obesity, family history of diabetes) should have their fasting blood glucose monitored before beginning treatment and periodically during treatment. Patients who develop symptoms of hyperglycemia should have assessments of fasting glucose. Clinical monitoring of weight is recommended [see Warnings and Precautions (.
- Interference with Cognitive and Motor Performance: Because CLOZARIL may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that CLOZARIL therapy does not affect them adversely [see Warnings and Precautions (.
- Missed Doses and Re-initiating Treatment: Inform patients and caregivers that if the patient misses taking CLOZARIL for 1 day or more, they should not restart their medication at the same dosage but should contact their physician for dosing instructions [see Dosage and Administration (.
- Pregnancy: Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with CLOZARIL. Advise patients that CLOZARIL may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CLOZARIL during pregnancy [see Use in Specific Populations (.
- Lactation: Advise breastfeeding women using CLOZARIL to monitor infants for excess sedation and to seek medical care if they notice this sign. Inform breastfeeding women using CLOZARIL that their healthcare provider will monitor infants for neutropenia[see Use in Specific Populations (
- Concomitant Medication: Advise patients to inform their healthcare provider if they are taking, or plan to take, any prescription or over-the-counter drugs; there is a potential for significant drug-drug interactions [see Dosage and Administration (.
6MEDICATION GUIDE
CLOZARIL (klow-zr-uhl)
(clozapine)
tablets, for oral use
(clozapine)
tablets, for oral use
What is the most important information I should know about CLOZARIL?
CLOZARIL can cause serious side effects including:
- Severe neutropenia (low white blood cell (WBC) counts) that can lead to serious infections and death.
- Your healthcare provider will do WBC blood tests before starting treatment with CLOZARIL and weekly for the first 6 months. After your first 6 months of treatment, your healthcare provider will determine how frequent you will have blood tests. If you have symptoms of severe neutropenia or an infection, your healthcare provider will do more frequent WBC blood test(s) to check if CLOZARIL is causing your symptoms and may send you to see a blood specialist (hematologist). Tell your health care provider right away if you have any of the following symptoms or signs of neutropenia or infection:
- Orthostatic hypotension (decreased blood pressure), bradycardia (slow heart rate), or syncope (fainting) that can lead to death. You may feel lightheaded or faint when you rise too quickly from a sitting or lying position. Tell your healthcare provider right away if you feel dizzy or pass out.
- Seizures. See “What should I avoid while taking CLOZARIL?”
- Myocarditis (heart muscle inflammation), pericarditis (inflammation of outer layer of the heart) and cardiomyopathy (heart muscle weakness) that can lead to death. Symptoms of myocarditis, pericarditis, and cardiomyopathy include:
- Increased risk of death in elderly people with dementia-related psychosis. Medicines like CLOZARIL can increase the risk of death in elderly people who have lost touch with reality (psychosis) due to confusion and dementia. CLOZARIL is not for treatment of elderly people with dementia-related psychosis.
What is CLOZARIL?
CLOZARIL is a prescription antipsychotic medicine used to treat people:
- Who are severely ill with schizophrenia not helped by other schizophrenia medicines
- With schizophrenia or schizoaffective disorder who have been suicidal and may be at risk of suicidal behavior again
It is not known if CLOZARIL is safe and effective in children.
Who should not take CLOZARIL?
Do not take CLOZARIL if you:
- are allergic to clozapine or any of the ingredients in CLOZARIL. See the end of this Medication Guide for a complete list of ingredients in CLOZARIL.
Before taking CLOZARIL, tell your healthcare provider about all your medical conditions, including if you:
- have or have had heart problems or a family history of heart problems including heart attack, heart failure, abnormal heart rhythm or long QT syndrome, or stroke
- have or have had low or high blood pressure
- have or have had kidney or liver problems
- have or have had seizures (convulsions)
- have or have had stomach or intestinal problems including constipation, slow emptying of your stomach, or diarrhea
- have or have had low levels of potassium or magnesium in your blood
- have or have had diabetes or high blood sugar in you or your family
- have or have had high levels of total cholesterol, “bad” cholesterol (LDL-C), or triglycerides, or low levels of “good” cholesterol (HDL-C)
- have increased pressure in your eyes (glaucoma), an enlarged prostate, or problems passing urine
- have or have had uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia)
- smoke tobacco
- plan to stop smoking tobacco while taking CLOZARIL
- use products containing caffeine
- are pregnant or plan to become pregnant. Talk to your healthcare provider if you become pregnant while taking CLOZARIL.
- are breast feeding or plan to breast feed. CLOZARIL can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take CLOZARIL.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
- CLOZARIL and other medicines may affect each other causing side effects.
- Your healthcare provider can tell you if it is safe to take CLOZARIL with your other medicines. Do not start or stop any medicines while taking CLOZARIL without talking to your healthcare provider first.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take CLOZARIL?
- Take CLOZARIL exactly as your healthcare provider tells you to take it.
- Take CLOZARIL with or without food.
- If you miss taking CLOZARIL for 1 day or more, call your healthcare provider right away. Do not take 2 doses at the same time unless your healthcare provider tells you to.
- If you take too much (overdose) CLOZARIL, call your healthcare provider or the Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
- Symptoms of CLOZARIL overdose can include:
What should I avoid while taking CLOZARIL?
- You should not drink alcohol while taking CLOZARIL because it can increase your chances of getting serious side effects.
- Do not drive, operate machinery, swim, climb, or do dangerous activities until you know how CLOZARIL affects you.
What are the possible side effects of CLOZARIL?
CLOZARIL can cause serious side effects, including:
- See
- falls. CLOZARIL may make you sleepy, dizzy, may cause a decrease in your blood pressure when changing positions, and can slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries.
- slow emptying of your stomach and intestines (decreased gastric motility). Severe constipation and bowel problems can happen and can lead to hospitalization, surgery, and death. You may not feel or be aware of constipation symptoms. Your healthcare provider will examine you for possible bowel problems. Tell your healthcare provider if you get any signs and symptoms of decreased gastrointestinal motility during treatment with CLOZARIL, including:
- Staying well hydrated, increasing physical activity, and taking fiber during treatment with CLOZARIL can help prevent constipation and other bowel problems. Your healthcare provider may prescribe medicines to prevent severe problems.
- high count of a certain white blood cell (eosinophilia). CLOZARIL can cause a high count of eosinophils in some people and can be serious. This is a different risk than the risk of CLOZARIL causing an abnormally low white blood cell count (neutropenia). Your health care provider may send you to see an internal medicine specialist (internist) or blood specialist (hematologist). Tell your healthcare provider right away if you have any of these symptoms:
- serious heart rhythm problems (QTc Interval Prolongation) that can cause death. Your healthcare provider will do a physical exam and may obtain blood tests and an electrocardiogram before starting you on treatment with CLOZARIL. Tell your healthcare provider right away if you have any of these symptoms:
- passing out or feeling like you will pass out
- dizziness
- feeling as if your heart is pounding or missing beats
- problems with your metabolism such as:
- high blood sugar (hyperglycemia) or diabetes. Increases in blood sugar can happen in some people who take CLOZARIL. Extremely high blood sugar can lead to coma and death. If you have diabetes or risk factors for diabetes (such as being overweight), your health care provider should check your blood sugar before you start CLOZARIL and during treatment. Tell your healthcare provider if you have any of these symptoms of high blood sugar while taking CLOZARIL:
- increased fat levels (cholesterol and triglycerides) in your blood (dyslipidemia). Your healthcare provider should check the fat levels in your blood before you start and during treatment with CLOZARIL.
- weight gain. You and your healthcare provider should check your weight regularly.
- neuroleptic malignant syndrome (NMS). NMS is a rare but serious condition that can lead to death and must be treated in a hospital. Tell your healthcare provider right away if you become severely ill and have any of these symptoms:
- liver problems. CLOZARIL can cause serious life-threatening liver problems that can lead to death. Tell your healthcare provider right away if you have any of these symptoms:
- fever. Some people may have a fever while they take CLOZARIL. If you have a fever, your healthcare provider will do blood tests to check for neutropenia or an infection. Your healthcare provider may also send you to see a blood specialist (hematologist). Tell your healthcare provider if you have a fever.
- blood clot in your lung (pulmonary embolism) or in the veins of your legs (deep vein thrombosis). Get emergency help right away if you have symptoms of a blood clot including:
- chest pain and shortness of breath
- swelling or pain in your leg, ankle or foot
- warm feeling in the skin of your affected leg
- changes in your skin color such as turning pale or blue
- a problem that includes dry mouth, increased sweating, increased pulse rate, constipation, and urinary retention (anticholinergic toxicity).
- problems thinking clearly and moving your body. See “What should I avoid while taking CLOZARIL?”
- uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia). Tardive dyskinesia may not go away, even if you stop CLOZARIL. Tardive dyskinesia may also start after you stop taking CLOZARIL.
- stroke (cerebrovascular problems) in elderly people with dementia-related psychosis that can lead to death.
The most common side effects of CLOZARIL include:
- These are not all the possible side effects of CLOZARIL.
- Your healthcare provider may lower your dose or temporarily or permanently stop treatment with CLOZARIL if you have certain symptoms or if your WBC count is low.
- Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
- You may report side effects to FDA at 1-800-FDA-1088.
How should I store CLOZARIL?
- Store CLOZARIL at room temperature between 68°F to 77°F (20°C to 25°C).
Keep CLOZARIL and all medicines out of the reach of children.
General information about the safe and effective use of CLOZARIL.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use CLOZARIL for a condition for which it was not prescribed. Do not give CLOZARIL to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider (including pharmacist) for information about CLOZARIL that is written for health professionals.
What are the ingredients in CLOZARIL?
Active ingredients: clozapine
Inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, povidone, starch (corn), and talc
Distributed by: HLS Therapeutics (USA), Inc., Rosemont, PA 19010, (844) 457-8721
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: 06/2025
MLR USA: 20250601 associated with 2025HER001
7Package/Label Display Panel
Package Label – 25 mg
Rx Only NDC 69809-0126-05
CLOZARIL
100 Tablets

8Package/Label Display Panel
Package Label – 100 mg
Rx Only NDC 69809-0127-05
CLOZARIL
100 Tablets
