Brand Name
Kengreal
Generic Name
Cangrelor
View Brand Information FDA approval date: July 08, 2015
Classification: P2Y12 Platelet Inhibitor
Form: Injection
What is Kengreal (Cangrelor)?
Cangrelor for injection is indicated as an adjunct to percutaneous coronary intervention to reduce the risk of periprocedural myocardial infarction , repeat coronary revascularization, and stent thrombosis in patients who have not been treated with a P2Y 12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor [see Clinical Studies (1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
KENGREAL (cangrelor)
1INDICATIONS AND USAGE
KENGREAL is indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y
2DOSAGE FORMS AND STRENGTHS
For Injection: 50 mg of KENGREAL lyophilized powder in a single-use 10 mL glass vial for reconstitution.
3ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
- Bleeding
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of KENGREAL has been evaluated in 13,301 subjects in controlled trials, in whom, 5,529 were in the CHAMPION PHOENIX trial.
Bleeding
There was a greater incidence of bleeding with KENGREAL than with clopidogrel. No baseline demographic factor altered the relative risk of bleeding with KENGREAL (see Table 1 and Figure 1).
Abbreviations: GUSTO: Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries; TIMI: Thrombolysis in Myocardial Infarction
a Safety population is all randomized subjects who received at least one dose of study drug
b intracranial hemorrhage or bleeding resulting in substantial hemodynamic compromise requiring treatment
c requiring blood transfusion but not resulting in hemodynamic compromise
d all other bleeding not included in severe or moderate
e any intracranial hemorrhage, or any overt bleeding associated with a reduction in hemoglobin of ≥5 g/dL (or, when hemoglobin is not available, an absolute reduction in hematocrit ≥15%)
f any overt sign of bleeding (including observation by imaging techniques) that is associated with a reduction in hemoglobin of ≥3 g/dL and <5 g/dL (or, when hemoglobin is not available, an absolute reduction in hematocrit of ≥9% and <15%)
Figure 1:Bleeding Results in the CHAMPION PHOENIX Studya (All Non-CABG related)
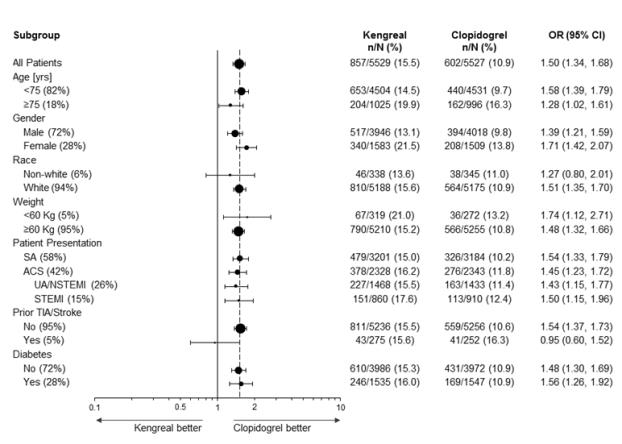
a Safety population is all randomized subjects who received at least one dose of study drug
Note: The figure above presents effects in various subgroups most of which are baseline characteristics and most of which were pre-specified (patient presentation and weight were not pre-specified subgroups). The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
Drug Discontinuation
In CHAMPION PHOENIX the rate of discontinuation for bleeding events was 0.3% for KENGREAL and 0.1% for clopidogrel. Discontinuation for non-bleeding adverse events was low and similar for KENGREAL (0.6%) and for clopidogrel (0.4%). Coronary artery dissection, coronary artery perforation, and dyspnea were the most frequent events leading to discontinuation in patients treated with KENGREAL.
Non-Bleeding Adverse Reactions
Hypersensitivity
Serious cases of hypersensitivity were more frequent with KENGREAL (7/13301) than with control (2/12861). These included anaphylactic reactions, anaphylactic shock, bronchospasm, angioedema, and stridor.
Decreased renal function
Worsening renal function was reported in 3.2% of KENGREAL patients with severe renal impairment (creatinine clearance <30 mL/min) compared to 1.4% of clopidogrel patients with severe renal impairment.
Dyspnea
Dyspnea was reported more frequently in patients treated with KENGREAL (1.3%) than with control (0.4%).
4OVERDOSAGE
There is no specific treatment to reverse the antiplatelet effect of KENGREAL but the effect is gone within one hour after the drug is discontinued.
In clinical trials, 36 patients received an overdose of KENGREAL, ranging from 36 to 300 mcg/kg (bolus dose) or 4.8 to 13.7 mcg/kg/min (infusion dose). The maximum overdose received was 10 times the PCI bolus dose or 3.5 times the PCI infusion dose in 4 patients. No clinical sequela were noted as a result of overdose following completion of KENGREAL therapy.
5DESCRIPTION
KENGREAL is a direct-acting P2Y
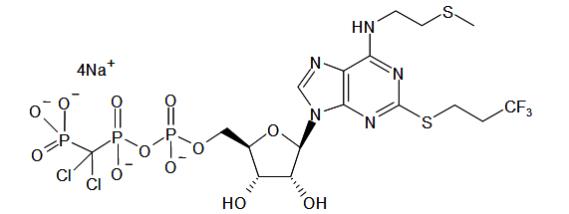
The chemical name of KENGREAL is tetrasodium salt of N6-[2-(methylthio)ethyl]-2-[(3,3,3,-trifluoropropyl)-5’-adenylic acid, monanhydride with (dichloromethylene) bisphosphonic acid.
The empirical formula of KENGREAL is C
The chemical structure is represented below:
Cangrelor for Injection is a sterile white to off-white lyophilized powder for IV infusion. In addition to the active ingredient, cangrelor, each single use vial contains mannitol (158 mg), sorbitol (52 mg), and sodium hydroxide to adjust the pH.
6HOW SUPPLIED/STORAGE AND HANDLING
KENGREAL is supplied as a sterile lyophilized powder in single-use 10 mL vials.
- NDC # 10122-620-01: 10 mL vial containing 50 mg cangrelor
- NDC # 10122-620-10: 10 count of 10 mL vials containing 50 mg cangrelor
Vials of KENGREAL should be stored at USP Controlled Room Temperature, [20°C to 25°C (68°F to 77°F) with excursions between 15°C and 30°C (59°F and 86°F) permitted].
KENGREAL
Distributed by:
US-126-3-SPL