Brand Name
Fenofibric
View Brand InformationFDA approval date: December 04, 2013
Classification: Peroxisome Proliferator Receptor alpha Agonist
Form: Capsule
What is Fenofibric?
Fenofibric acid delayed-release capsules are a peroxisome proliferator-activated receptor alpha agonist indicated as adjunctive therapy to diet to: Reduce TG in patients with severe hypertriglyceridemia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Fenofibric acid (Fenofibric acid)
1ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Mortality and coronary heart disease morbidity
- Hepatoxicity
- Pancreatitis
- Hypersensitivity reactions
- Venothromboembolic disease
1.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
1.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fenofibrate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: rhabdomyolysis, pancreatitis, renal failure, muscle spasms, acute renal failure, hepatitis, cirrhosis, increased total bilirubin, anemia, asthenia, severely depressed HDL-cholesterol levels, and interstitial lung disease. Photosensitivity reactions to fenofibrate have occurred days to months after initiation; in some of these cases, patients reported a prior photosensitivity reaction to ketoprofen.
2OVERDOSAGE
There is no specific treatment for overdose with fenofibric acid. General supportive care of the patient is indicated, including monitoring of vital signs and observation of clinical status, should an overdose occur. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. Because fenofibric acid is highly bound to plasma proteins, hemodialysis should not be considered.
3DESCRIPTION
Fenofibric acid is a lipid regulating agent available as delayed release capsules for oral administration. Each delayed-release capsule contains choline fenofibrate, equivalent to 45 mg or 135 mg of fenofibric acid. The chemical name for choline fenofibrate is ethanaminium, 2-hydroxy-N,N,N-trimethyl, 2-{4-(4-chlorobenzoyl)phenoxy] -2-methylpropanoate (1:1) with the following structural formula:
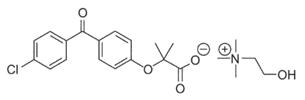
The empirical formula is C
Each delayed-release capsule contains enteric coated mini-tablets comprised of choline fenofibrate and the following inactive ingredients: colloidal silicon dioxide, dibutyl sebacate, ethyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, methacrylic acid co polymer, povidone, sodium stearyl fumarate, talc and triethyl citrate. The capsule shell of the 45 mg capsule contains the following inactive ingredients: gelatin, iron oxide black, iron oxide red, iron oxide yellow, sodium lauryl sulphate and titanium dioxide. The capsule shell of the 135 mg capsule contains the following inactive ingredients: FD and C Blue #1, gelatin, iron oxide yellow, sodium lauryl sulphate and titanium dioxide. The capsules are printed with edible ink containing iron oxide black, potassium hydroxide, propylene glycol and shellac.
4HOW SUPPLIED/STORAGE AND HANDLING
Fenofibric acid delayed-release capsules are supplied in two dose strengths as follows:
- Fenofibric acid delayed-release capsules, 45 mg are size '3' capsule with brown cap and yellow body, imprinted with "LU" on cap and "Q41" on body in black ink, containing four white to off white mini-tablets. The delayed-release capsules are available in bottles of 90's (NDC 68180-128-09); 100's (NDC 68180-128-01) and 500's (NDC 68180-128-02).
- Fenofibric acid delayed-release capsules, 135 mg are size '0' capsule with blue opaque cap and yellow opaque body, imprinted with "LU" on cap and "Q42" on body in black ink, containing twelve white to off white mini-tablets. The delayed-release capsules are available in bottle of 90's (NDC 68180-129-09); 100's (NDC 68180-129-01) and 500's (NDC 68180-129-02).
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F). [see USP Controlled Room Temperature]. Keep out of the reach of children. Protect from moisture.
5PATIENT COUNSELING INFORMATION
Patients should be advised:
- of the potential benefits and risks of fenofibric acid delayed-release capsules.
- not to use fenofibric acid delayed-release capsules if there is a known hypersensitivity to fenofibrate or fenofibric acid.
- of medications that should not be taken in combination with fenofibric acid delayed-release capsules.
- that if they are taking coumarin anticoagulants, fenofibric acid delayed-release capsules may increase their anti-coagulant effect, and increased monitoring may be necessary.
- to continue to follow an appropriate lipid-modifying diet while taking fenofibric acid delayed-release capsules.
- to take fenofibric acid delayed-release capsules once daily, without regard to food, at the prescribed dose, swallowing each capsule whole.
- to return to their physician's office for routine monitoring.
- to inform their physician of all medications, supplements, and herbal preparations they are taking and any change to their medical condition. Patients should also be advised to inform their physicians prescribing a new medication that they are taking fenofibric acid delayed-release capsules.
- to inform their physician of symptoms of liver injury (e.g., jaundice, abdominal pain, nausea, malaise, dark urine, abnormal stool, pruritus); any muscle pain, tenderness, or weakness; or any other new symptoms.
- not to breastfeed during treatment with fenofibric acid delayed-release capsules and for 5 days after the final dose.
* The brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.
Manufactured for
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Goa - 403 722
INDIA
Revised: July 2021 ID#: 268317
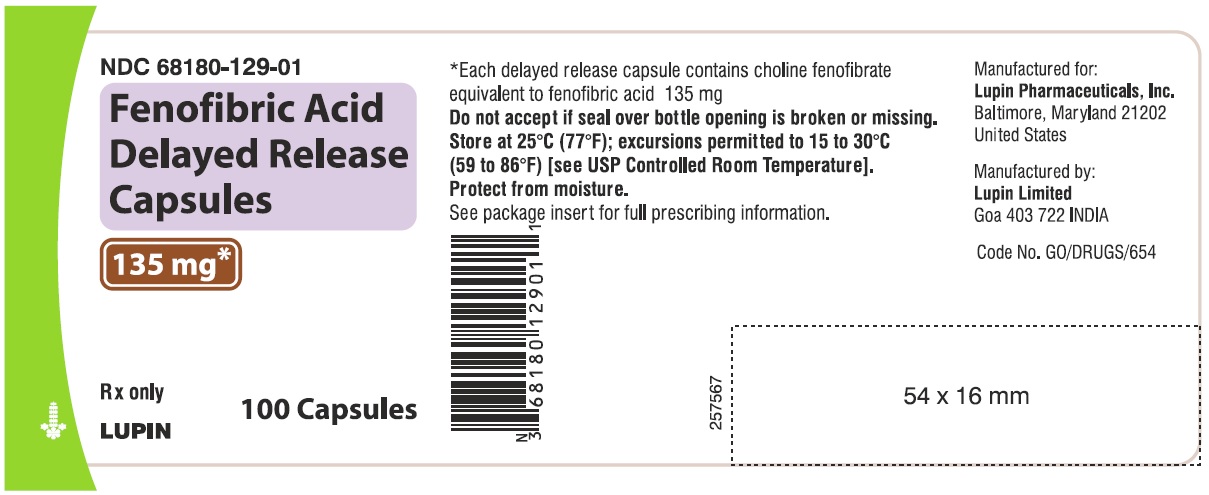
6PACKAGE LABEL PRINCIPAL DISPLAY PANEL
FENOFIBRIC ACID DELAYED-RELEASE CAPSULES
Rx Only
45 mg
NDC 68180-128-01
100 Tablets

FENOFIBRIC ACID DELAYED-RELEASE CAPSULES
Rx Only
135 mg
NDC 68180-129-01
100 Tablets