Opsumit
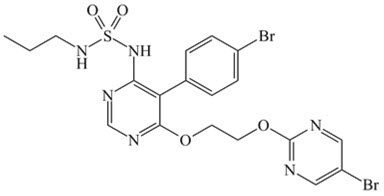
What is Opsumit (Macitentan)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of the study is to enable participants with pulmonary hypertension (PH) currently treated with study intervention(s) in a clinical study (parent studies \[NCT03422328, NCT03904693,NCT04565990, NCT02932410, NCT03492177, and NCT04175600\]), to continue to benefit from the intervention after closure of the parent study in case they have no alternative means of access to the study interven...
Summary: The main goal of this study is to determine the effects of combination medical therapy (Riociguat and Macitentan) and balloon pulmonary angioplasty (BPA) on hemodynamics and right ventricular (RV) function (including advanced assessments of RV-pulmonary artery (PA) coupling from invasive hemodynamics) in participants with inoperable or post-PTE residual CTEPH.
Summary: Coronary microvascular angina (MVA) significantly reduces quality of life and increases the risk of heart problems in patients with angina. Unfortunately, there are no effective treatments available yet. The endothelin-1 (ET-1) - endothelin receptor (ETRs) system plays a critical role in MVA. Preclinical studies demonstrate that ETRs antagonists or pericyte-specific knockdown of ETRs can improve c...
Related Latest Advances
Brand Information
- Embryo-fetal Toxicity
- Hepatotoxicity
- Fluid Retention
- Decrease in Hemoglobin