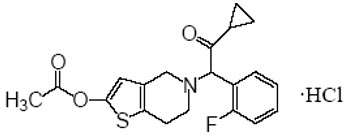
Effient
What is Effient (Prasugrel)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: DAPT de-escalation strategies to reduce bleeding include de-escalation of DAPT intensity (downgrading from potent P2Y12 inhibitor at conventional doses to either clopidogrel or reduced-dose prasugrel) or abbreviation of DAPT duration. The EASTYLE trial will evaluate a hybrid DAPT de-escalation strategy (reduced-dose ticagrelor, followed by aspirin early discontinuation) in AMI patients, compared w...
Summary: The study is a multi-centre, Open-label, Randomized Controlled, 1:1 trial comparing Prasugrel-based short DAPT (30-45 days) followed by Prasugrel monotherapy versus standard DAPT regimen in STEMI patients in terms of safety and efficacy endpoints. In the subgroup of STEMI patients with MVD, a sub-randomization will allow a comparison between a complete revascularization OCT-guided versus complete ...
Summary: Factorial 2x2, all-comer, multicentre, randomized controlled trial (ratio 1:1:1:1). First, the study will compare (first randomization) the non-inferiority in target lesion failure of angiolite stent versus Xience stent family. Immediately after the first randomization, the study compares (second randomization) the superiority in bleeding Bleeding Academic Research Consortium (BARC) 2, 3, or 5 of ...
Related Latest Advances
Brand Information
- Effient can cause significant, sometimes fatal, bleeding
- Do not use Effient in patients with active pathological bleeding or a history of transient ischemic attack (TIA) or stroke
- In patients ≥75 years of age, Effient is generally not recommended, because of the increased risk of fatal and intracranial bleeding and uncertain benefit, except in high-risk situations (patients with diabetes or a history of prior myocardial infarction [MI]) where its effect appears to be greater and its use may be considered
- Do not start Effient in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue Effient at least 7 days prior to any surgery
- Additional risk factors for bleeding include: body weight <60 kg, propensity to bleed, concomitant use of medications that increase the risk of bleeding (e.g
- Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of Effient
- If possible, manage bleeding without discontinuing Effient. Discontinuing Effient, particularly in the first few weeks after acute coronary syndrome, increases the risk of subsequent cardiovascular (CV) events
- Bleeding
- Thrombotic Thrombocytopenic Purpura
- Hypersensitivity Including Angioedema