Brand Name
Moxidectin
View Brand InformationFDA approval date: December 02, 2019
Form: Tablet
What is Moxidectin?
Moxidectin Tablets are indicated for the treatment of onchocerciasis due to Onchocerca volvulus in adult and pediatric patients aged 4 years and older and weighing at least 13 kg. Limitations of Use: Moxidectin Tablets does not kill adult Onchocerca volvulus parasites. Follow-up evaluation is advised. The safety and efficacy of repeat administration of Moxidectin Tablets in patients with O. volvulus has not been studied. Moxidectin is an anthelmintic indicated for the treatment of onchocerciasis due to Onchocerca volvulus in adults and pediatric patients aged 4 years and older and weighing at least 13 kg. Limitations of Use: Moxidectin Tablets do not kill adult Onchocerca volvulus parasites. Follow-up is advised. The safety and efficacy of repeat administration of Moxidectin Tablets in patients with O. volvulus has not been studied.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Moxidectin Versus Ivermectin as Mass Drug Administration for the Control of Onchocerciasis and Other Neglected Tropical Diseases: A Cluster-randomised Trial
Summary: This clinical trial compares two treatments - ivermectin and moxidectin - to learn which is better at reducing the proportion of people with onchocerciasis (river blindness) when given through mass drug administration (MDA) in Angola. Both drugs are approved by the United States Food and Drug Administration (FDA) to treat this disease. The study also explores how these treatments affect other infe...
Related Latest Advances
Brand Information
Moxidectin (Moxidectin)
1INDICATIONS AND USAGE
Moxidectin Tablets are indicated for the treatment of onchocerciasis due to
Limitations of Use:
Moxidectin Tablets does not kill adult
The safety and efficacy of repeat administration of Moxidectin Tablets in patients with
2DOSAGE FORMS AND STRENGTHS
Moxidectin Tablets: 2 mg of moxidectin, white to pale yellow, uncoated, oval-shaped, debossed on one side with “AKKA”.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described in greater detail in other labeling sections:
- Cutaneous, Ophthalmological and/or Systemic Adverse Reactions
- Symptomatic Orthostatic Hypotension
- Encephalopathy in
- Edema and Worsening of Onchodermatitis
4.1Clinical Trials Experience
Because clinical trials are conducted under varying controlled conditions, adverse reaction rates observed in one clinical trial cannot be directly compared to rates observed in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinical Trials Experience in Adults and Pediatric Patients (12 Years to Less than 18 Years of Age)
The safety of Moxidectin Tablets was evaluated in two randomized, double-blind, active-controlled studies in patients with confirmed onchocerciasis (Trial 1 and Trial 2)
Most Common Adverse Reactions
No patients withdrew from either trial due to adverse reactions. Adverse Reactions reported in Trial 1 in > 10% of patients are summarized in Table 2. Most were related to physical, vital signs and laboratory changes associated with Mazzotti reaction
aIncludes “myalgia”, “arthralgia”, “musculoskeletal pain”, “pain” and “back pain”
bIncludes “orthostatic heart rate increased”, “postural orthostatic tachycardia syndrome”, “heart rate increased” and “sinus tachycardia”
cIncludes “orthostatic heart rate increased” and “postural orthostatic tachycardia syndrome”
dIncludes “heart rate increased”, “tachycardia”, and “sinus tachycardia”
eIncludes “rash,” “papular rash” and “urticaria”
fIncludes “abdominal pain”, “abdominal pain upper” and “abdominal pain lower”
gIncludes “orthostatic hypotension”, “blood pressure orthostatic decreased”, “blood pressure decreased”, “mean arterial pressure decreased”, “hypotension”
hIncludes “orthostatic hypotension”, and “blood pressure orthostatic decreased”
*Lymphocytopenia is defined as absolute lymphocyte count less than 1 x 10
**Neutropenia is defined as absolute neutrophil count less than 1 x 10
The most common adverse reactions in patients (N = 38) treated with 8 mg moxidectin in Trial 2 were similar to the adverse reactions noted in Trial 1 described in Table 2above.
OtherAdverse Reactions Reported in Clinical Trials(Trial 1 and Trial 2)
The following adverse reactions occurred in less than 10% of subjects receiving Moxidectin Tablets in Trial 1:
OcularAdverse Reactions:In Trial 1, the most common ocular adverse reactions (occurring in ≥ 0.5% of patients) is shown in Table 3.
*Includes “visual impairment”, “blurred vision” and “low vision acuity”
**Includes “foreign body sensation”, “ocular discomfort” and “abnormal sensation in the eye”
Hepatobiliary Adverse Reactions
More patients in the moxidectin arm experienced elevation in bilirubin above the upper limit of normal and elevation in transaminases > 5x upper limit of normal. Twenty-seven (2.8%) patients in the moxidectin arm and 3 (0.6%) patients in the ivermectin arm had hyperbilirubinemia. Most of the patients had single measurements of hyperbilirubinemia without concurrent elevation in transaminases.
Nine (1%) patients in the moxidectin arm and 2 (0.4%) patients in the ivermectin arm had elevation in ALT of more than 5x upper limit of normal; ten (1%) patients in the moxidectin arm and 3 (0.6%) patients in the ivermectin arm had elevation in AST to more than 5x upper limit of normal.
Laboratory Abnormalities
Laboratory abnormalities occurring in at least 1% of patients in Trial 1 are described in Table 4.
Clinical Trials Experience in Pediatric Patients (4 Years to Less than 18 Years of Age)
The safety of Moxidectin Tablets in pediatric patients is based on data from 2 studies, Trial 1 and Trial 3 (NCT01035619).
Pediatric Patients in Trial 1
Trial 1 included 53 pediatric patients aged 12 to less than 18 years with confirmed onchocerciasis who received a single dose of Moxidectin Tablets 8 mg. Pediatric patients with confirmed infection experienced efficacy-related adverse reactions (Mazzotti reactions) such as abdominal pain, tachycardia, pyrexia, rash, peripheral swelling, and lymph node pain at a prevalence and severity similar to infected adults. Overall, the safety profile relative to age was similar across pediatric and adult patients studied in Trial 1.
Pediatric Patients in Trial 3
Trial 3 was a single-arm, open-label, age-stratified, multi-cohort, safety and pharmacokinetic trial that evaluated 36 pediatric patients aged 4 to less than 18 years with unknown O. volvulus infection status from an onchocerciasis-endemic area in Ghana. Patients were stratified by age to receive a single dose of Moxidectin Tablets as follows: aged 12 to less than 18 years received 8 mg (N = 9), 8 to less than 12 years received 8 mg (N = 9) or 6 mg (N = 9), and 4 to less than 8 years received 4 mg (N = 9). Median weight was 34.8 kg (range: 30.8 to 55.5 kg) in patients 12 to less than 18 years of age, 25.1 kg (range: 19.4 to 36.8 kg) in patients 8 to less than 12 years, and 15.6 kg (range: 13.6 to 20.6 kg) in patients 4 to less than 8 years. A majority of patients were female (58%) and 100% were black. Mazzotti reactions were not seen in this population with unknown O. volvulus infection. The most common adverse reactions in these pediatric patients were abdominal pain and diarrhea, in 3/36 (8%) patients each. No new safety signals were noted in this pediatric patient population that were not already noted in Trial 1.
5OVERDOSAGE
No specific antidote is available for overdose with Moxidectin Tablets. If overdose occurs, the patient should be monitored for evidence of toxicity. Treatment of overdose with Moxidectin Tablets consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. Supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present.
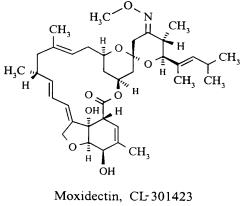
6DESCRIPTION
Moxidectin Tablets contain moxidectin, an anthelmintic drug and a macrocyclic lactone of the milbemycin class derived from the actinomycete
The chemical name of moxidectin is (2aE,4E,5'R,6R,6'S,8E,11R,13S,15S,17aR,20R,20aR,20bS)-6'-[(E)-1,3-dimethyl-1-butenyl]-5',6,6',7,10,11,14,15,17a,20,20a,20b-dodecahydro-20,20b-dihydroxy-5',6,8,19-tetramethylspiro[11,15-methano-2H,13H,17H-furo[4,3,2-pq][2,6]benzodioxacyclooctadecin-13,2'-[2H]pyran]-4',17(3'H)-dione 4'-(E)-(O-methyloxime). The structural formula is:

Figure 1: Moxidectin Structure
Moxidectin is a white or pale-yellow, amorphous powder. The empirical formula is C
Moxidectin Tablets are for oral administration. Each tablet contains 2 mg of moxidectin. The tablets are uncoated and include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose anhydrous, magnesium stearate, microcrystalline cellulose and sodium lauryl sulfate.
7CLINICAL STUDIES
The assessment of the safety and efficacy of Moxidectin Tablets 8 mg in the treatment of onchocerciasis is based on data from two randomized, double-blind, active-controlled trials in patients with
Efficacy was assessed by skin microfilarial density (microfilariae/mg skin) from the mean of 4 skin snips per person per time point up to 18 months post-treatment.
Trial 1 recruited adult and pediatric patients ≥ 12 years with a body weight ≥ 30 kg and ≥ 10 microfilariae/mg skin. Mean (± SD) age was 42.5 (± 16.3) years, height 1.59 (± 0.09) meters, weight 51.6 (± 8.2) kg; 36.1% were female and 100% were black. Mean (± SD) pretreatment skin microfilarial density was 39.5 (± 30.7) microfilariae/mg skin, 69.6% had ≥ 20 microfilariae/mg skin and 39.7% had at least one ocular microfilaria.
Patients who were not previously exposed to ivermectin community directed treatment programs were recruited from the sub-Saharan African region (Democratic Republic of Congo, Liberia, and Ghana). Table 7 reports mean skin microfilarial density and the proportion of patients with undetectable skin microfilariae at Months 1, 6, and 12.
a Mean microfilarial density in skin is the average microfilarial density (microfilariae count/mg skin) over skin snips from four sites.
b Proportion of subjects undetectable (defined as a mean skin microfilariae density of zero across all 4 skin snips).
Additionally, safety and efficacy were assessed in a smaller single ascending dose trial (Trial 2, NCT 00300768) comparing 2 mg (n = 44), 4 mg (n = 45) (2 mg and 4 mg are not approved doses) and 8 mg (n = 38) single doses of moxidectin to ivermectin. Trial 2 was conducted in Ghana in adults aged ≥ 18 to ≤ 60 years with
8HOW SUPPLIED/STORAGE AND HANDLING
Moxidectin Tablets containing 2 mg moxidectin are white to pale yellow uncoated oval-shaped tablets, debossed on one side with “AKKA”. Each high-density polyethylene bottle contains 500 tablets (NDC 71705-050-01), a silica gel desiccant and rayon coil.
Store below 30°C (86°F).
- Protect from light.
- Once open, the full contents of the container should be used within 24 hours and discard any unused content.
9PATIENT COUNSELING INFORMATION
Signs and Symptoms Associated with Microfilarial Death
Advise patients that they are likely to have flu like symptoms including malaise, myalgia, headache, tachycardia, hypotension and pruritus, most commonly during the first week after treatment.
Symptomatic Orthostatic Hypotension
Advise patients that if they feel dizzy, faint or light-headed after taking Moxidectin Tablets, they should lie down until the symptoms resolve.
Absence of Macrofilarial Activity
Advise patients that treatment with Moxidectin Tablets does not kill adult
Edemaand Worseningof Onchodermatitis
Advise patients with hyper-reactive onchodermatitis that they may be more likely to experience severe adverse reactions.
Encephalopathy inLoa loaCo-infected Patients
Advise patients to report any symptoms of encephalopathy to their healthcare provider.
Lactation
Advise women that breastfeeding is not recommended at the time of treatment with Moxidectin Tablets and for 7 days after treatment.
Manufactured for: Medicines Development for Global Health, Melbourne, Victoria, Australia
© 2025 Medicines Development for Global Health. All rights reserved.
10PRINCIPAL DISPLAY PANEL
NDC 71705-050-01