Filgrastim
What is NYPOZI txid (Filgrastim)?
Approved To Treat
Related Clinical Trials
Summary: Idiopathic CD4 lymphocytopenia (ICL) is a rare syndrome defined by consistently low CD4 T cell counts (\<300/mm3) without evidence of HIV infection or other known immunodeficiency. Patients with ICL are at risk for opportunistic infections typically associated with HIV/AIDS such as disseminated cryptococcal infection and severe human papillomavirus-related dysplasia. More than 20 years since the d...
Summary: This phase II/III trial compares the effect of adding durvalumab to chemotherapy versus chemotherapy alone before surgery in treating patients with upper urinary tract cancer. Immunotherapy with monoclonal antibodies, such as durvalumab, may help the body's immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Chemotherapy drugs, such as methotrexat...
Background: \- White blood cells called granulocytes help the body fight infection. People who have had chemotherapy or bone marrow transplants may have very low numbers of these cells. Transfusions of these cells can help improve the body's ability to fight infection. However, most of the cells are located in the bone marrow or spleen, and are hard to collect from healthy donors. Two drugs, filgrastim and de...
Related Latest Advances
Brand Information
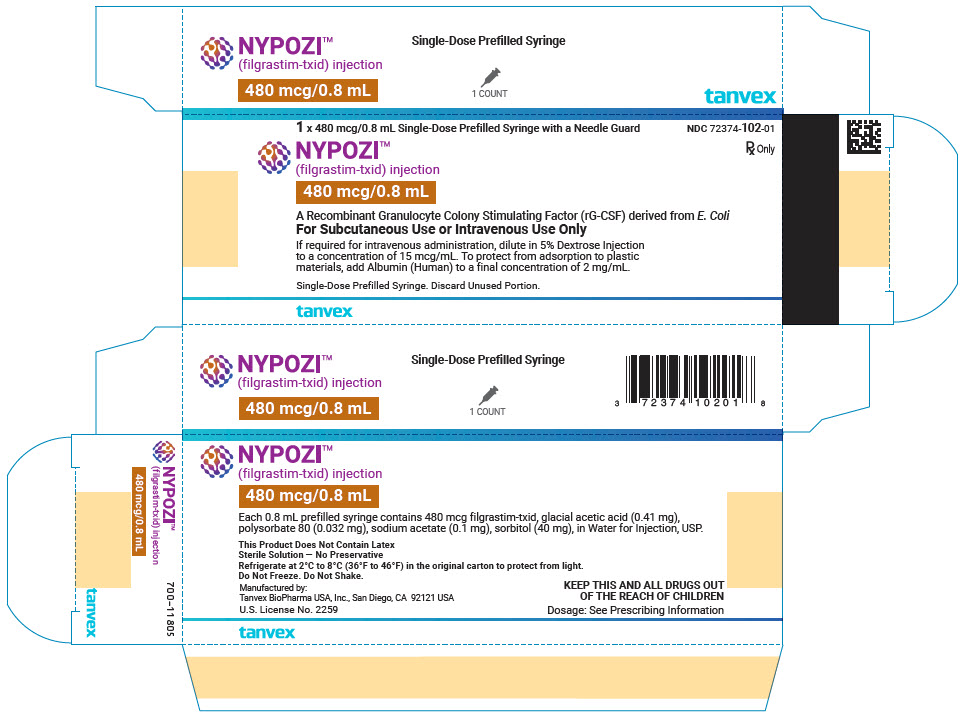
- Injection: 300 mcg/0.5 mL in a single-dose prefilled syringe with BD UltraSafe Passive™ Needle Guard
- Injection: 480 mcg/0.8 mL in a single-dose prefilled syringe with BD UltraSafe Passive™ Needle Guard
- Splenic Rupture
- Acute Respiratory Distress Syndrome
- Serious Allergic Reactions
- Sickle Cell Disorders
- Glomerulonephritis
- Alveolar Hemorrhage and Hemoptysis
- Capillary Leak Syndrome
- Myelodysplastic Syndrome
- Acute Myeloid Leukemia
- Thrombocytopenia
- Leukocytosis
- Cutaneous Vasculitis
- Aortitis
- splenic rupture and splenomegaly (enlarged spleen)
- acute respiratory distress syndrome
- anaphylaxis
- sickle cell disorders
- glomerulonephritis
- alveolar hemorrhage and hemoptysis
- capillary leak syndrome
- leukocytosis
- cutaneous vasculitis
- Sweet's syndrome (acute febrile neutrophilic dermatosis)
- decreased bone density and osteoporosis in pediatric patients receiving chronic treatment with filgrastim products
- myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with breast and lung cancer receiving chemotherapy and/or radiotherapy
- aortitis
- extramedullary hematopoiesis
- Carton of 1 prefilled syringe (NDC 72374-101-01)
- Carton of 10 prefilled syringes (NDC 72374-101-10)
- Carton of 1 prefilled syringe (NDC 72374-102-01)
- Carton of 10 prefilled syringes (NDC 72374-102-10)
- Rupture or enlargement of the spleen may occur. Symptoms include left upper quadrant abdominal pain or left shoulder pain. Advise patients to report pain in these areas to their physician immediately
- Dyspnea, with or without fever, progressing to Acute Respiratory Distress Syndrome, may occur. Advise patients to report dyspnea to their physician immediately
- Serious allergic reactions may occur, which may be signaled by rash‚ facial edema‚ wheezing‚ dyspnea‚ hypotension‚ or tachycardia. Advise patient to seek immediate medical attention if signs or symptoms of hypersensitivity reaction occur
- In patients with sickle cell disease, sickle cell crisis and death have occurred. Discuss potential risks and benefits for patients with sickle cell disease prior to the administration of human granulocyte colony-stimulating factors
- Glomerulonephritis may occur. Symptoms include swelling of the face or ankles, dark colored urine or blood in the urine, or a decrease in urine production. Advise patients to report signs or symptoms of glomerulonephritis to their physician immediately
- There may be an increased risk of Myelodysplastic Syndrome and/or Acute Myeloid Leukemia in patients with congenital neutropenia who receive filgrastim products and in patients with breast and lung cancer who receive filgrastim products in conjunction with chemotherapy and/or radiation therapy. Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding. Advise patients to report to their physician signs and symptoms of MDS/AML
- Cutaneous vasculitis may occur, which may be signaled by purpura or erythema. Advise patients to report signs or symptoms of vasculitis to their physician immediately
- Aortitis may occur. Symptoms may include fever, abdominal pain, malaise, back pain, and increased inflammatory markers. Advise patients to report signs and symptoms of aortitis to their physician immediately
- Importance of following the applicable Instructions for Use.
- Dangers of reusing needles or syringes.
- Importance of following local requirements for proper disposal of used syringes and needles.
- Importance of informing the healthcare provider if difficulty occurs when measuring or administering partial contents of the NYPOZI prefilled syringe.
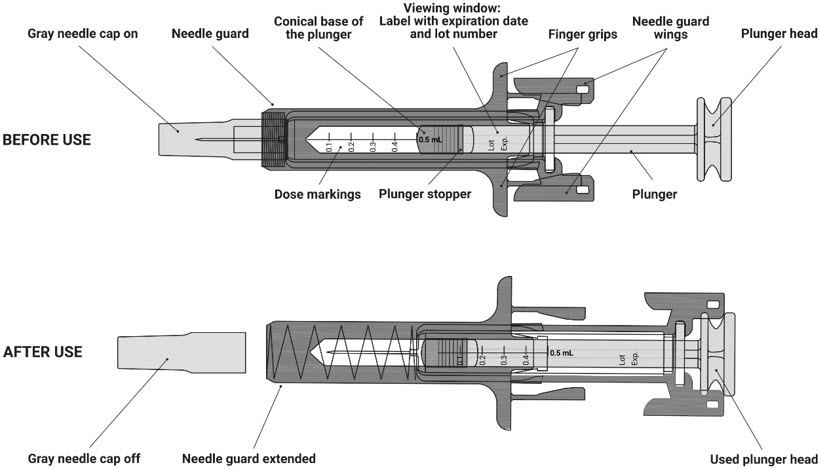
- Store the NYPOZI prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze NYPOZI prefilled syringes.
- Keep the prefilled syringe in the original carton to protect it from light or physical damage.
- Take the prefilled syringe out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- Throw away (dispose of) any prefilled syringe that has been left at room temperature for longer than 24 hours.
- After you inject your dose, throw away (dispose of) any unused NYPOZI left in the prefilled syringe.
- Keep the NYPOZI prefilled syringe out of the reach of children.
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- Make sure the name NYPOZI appears on the carton and prefilled syringe label.
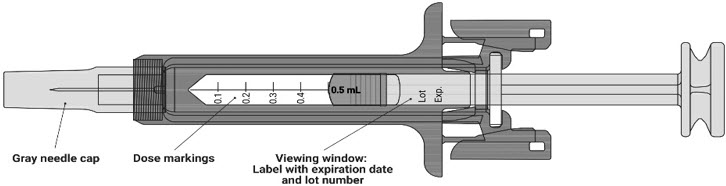
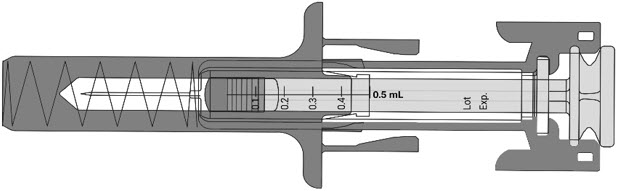
- Do not inject a dose of NYPOZI less than 0.3 mL (180 mcg) from a NYPOZI prefilled syringe. A dose less than 0.3 mL cannot be accurately measured using the NYPOZI prefilled syringe.
- Do not use a prefilled syringe after the expiration date on the label.
- Do not shake the prefilled syringe.
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
- Do not use the prefilled syringe if the carton is open or damaged.
- Do not use a prefilled syringe if it has been dropped on a hard surface. The prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe.
- The prefilled syringe has a needle guard that will be activated to cover the needle after the injection is given. The needle guard will help prevent needle stick injuries to anyone who handles the prefilled syringe.
- Avoid touching the syringe needle guard wings before use. Touching them may cause the syringe needle guard to be activated too early. Use another prefilled syringe that has not been activated and is ready to use.
- Do not use the prefilled syringe if the carton is damaged.
- Do not try to warm the prefilled syringe by using a heat source such as hot water or microwave.
- Keep the prefilled syringe in the blister pack until you are ready to use it.
- Do not leave the prefilled syringe in direct sunlight.
- Do not shake the prefilled syringe.
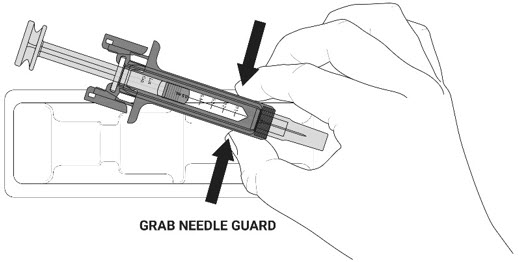
- Do not grab the plunger rod.
- Do not grab the gray needle cap.
- Do not use the prefilled syringe if:
- The medicine is cloudy or discolored or contains flakes or particles.
- Any part appears cracked or broken.
- The prefilled syringe has been dropped.
- The gray needle cap is missing or not securely attached.
- The expiration date printed on the label has passed.
- In all cases, use a new prefilled syringe and call your healthcare provider.
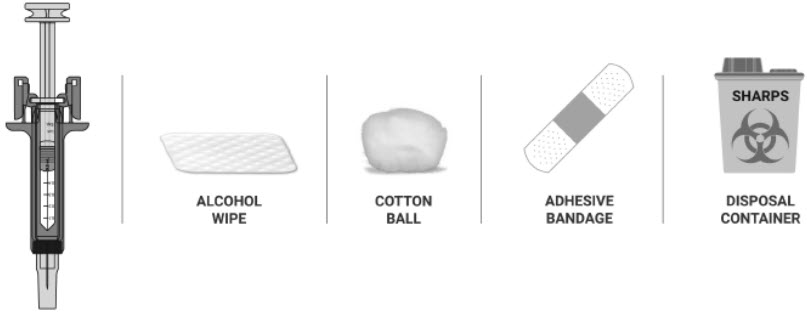
- Prefilled syringe, and the following items, that are not included in the NYPOZI carton
- Alcohol wipe
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container or other appropriate disposal container
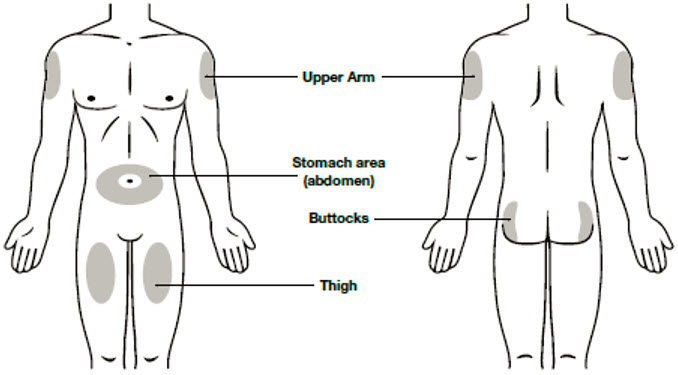
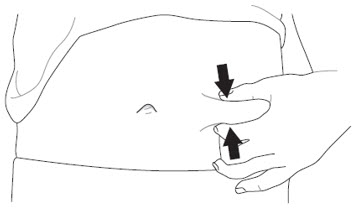
- Thigh
- Stomach area (abdomen), except for a
- Upper outer area of your buttocks (only if someone else is giving you the injection)
- Outer area of upper arm (only if someone else is giving you the injection)
- Let your skin dry.
- Do not touch this cleansed area again before injecting.
- Do not inject into areas where the skin is tender, bruised, red, scaly or hard. Avoid injecting into areas with scars or stretch marks.
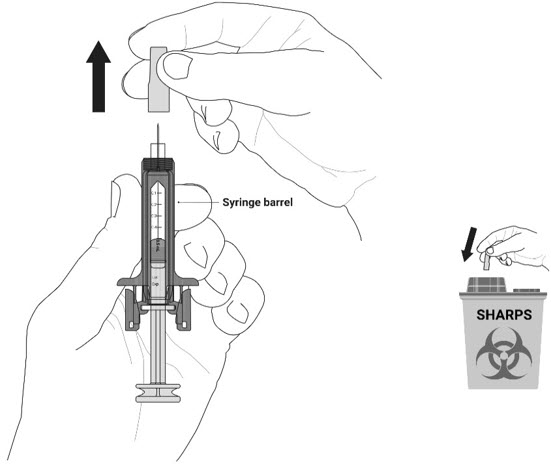
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
- Do not twist or bend the gray needle cap.
- Do not hold the prefilled syringe by the plunger rod.
- Do not put the gray needle cap back onto the prefilled syringe.
 Check your prescription before you inject your dose
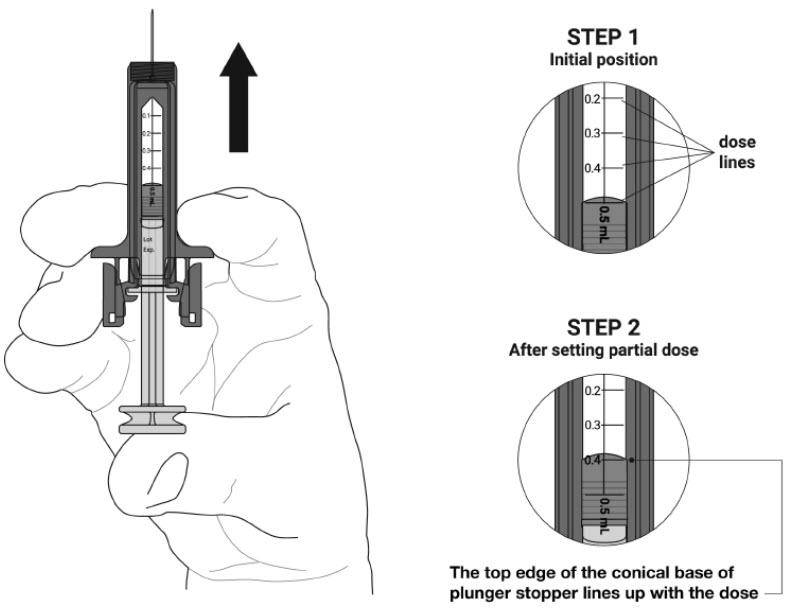
Check your prescription before you inject your dose- If you are prescribed a full dose, you will inject all of the medicine from your prefilled syringe.
- If you are prescribed a partial dose of NYPOZI, start with Step G below.
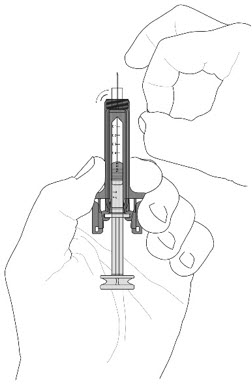
- See the figure below (
- Call your healthcare provider if you have problems measuring your prescribed dose.
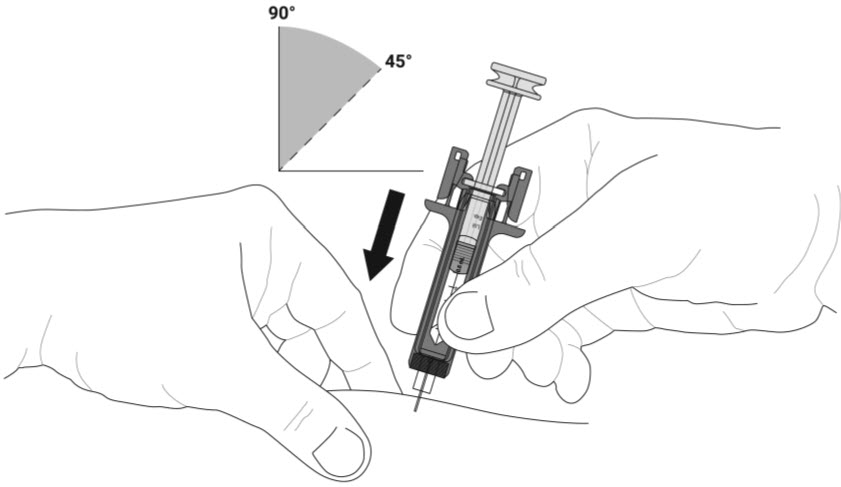
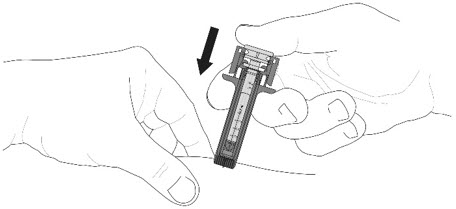
- Do not pull back the plunger rod while the needle is inserted.
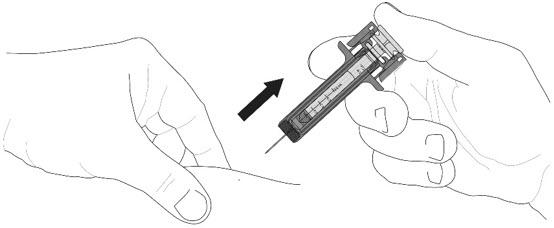

- Put the used prefilled syringe in a FDA-cleared sharps or other appropriate disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes, and prefilled syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not reuse the prefilled syringe.
- Do not recycle the prefilled syringe or sharps disposal container or throw them into household trash.
(filgrastim-txid) Injection