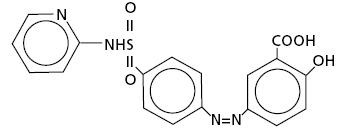
Sulfasalazine
What is Azulfidine EN-tabs (Sulfasalazine)?
Living with chronic inflammation, whether in the joints or the digestive tract, can deeply affect daily life. Conditions like rheumatoid arthritis and ulcerative colitis can cause persistent pain, fatigue, and discomfort that make even simple activities challenging. Sulfasalazine is a long-established medication that helps manage these inflammatory diseases, improving symptoms, and overall quality of life for many patients.
What does Sulfasalazine do?
Sulfasalazine is primarily used to treat inflammatory bowel disease (IBD) and certain types of arthritis. Doctors commonly prescribe it for:
- Ulcerative colitis, to reduce inflammation in the colon and relieve symptoms like diarrhea, abdominal pain, and bleeding.
- Rheumatoid arthritis (RA), to ease joint pain, stiffness, and swelling when other first-line treatments aren’t enough.
- Juvenile idiopathic arthritis (in children), in select cases.
In ulcerative colitis, sulfasalazine helps patients achieve and maintain remission, meaning fewer flares and more symptom-free days. In rheumatoid arthritis, it acts as a disease-modifying antirheumatic drug (DMARD), which means it not only eases symptoms but also slows joint damage over time.
Clinical studies have shown sulfasalazine to be effective in both gastrointestinal and joint inflammation management, particularly as part of combination therapy for rheumatoid arthritis (O’Dell et al., 1996).
How does Sulfasalazine work?
Sulfasalazine is a combination of two components, sulfapyridine and 5-aminosalicylic acid (5-ASA). When taken orally, gut bacteria break it down into these active parts:
- 5-ASA reduces inflammation in the colon lining, helping calm ulcerative colitis symptoms.
- Sulfapyridine contributes to immune-modulating effects, especially beneficial for arthritis.
Although the exact mechanism isn’t completely understood, sulfasalazine appears to suppress overactive immune responses and reduce the release of inflammatory substances like cytokines.
Clinically, this mechanism is valuable because it addresses both local gut inflammation and systemic immune activity, making the drug versatile in autoimmune conditions. By tackling inflammation at its source, sulfasalazine helps restore normal function and prevent long-term complications.
Sulfasalazine side effects
Sulfasalazine has been used for decades and is generally well-tolerated, but like all medications, it can cause side effects.
Common side effects:
- Nausea or upset stomach
- Loss of appetite
- Headache
- Skin rash
- Orange-yellow discoloration of urine or skin (harmless but notable)
Less common but serious side effects:
- Decreased white blood cells or platelets (which can increase infection or bleeding risk)
- Liver inflammation
- Allergic reactions such as fever, rash, or difficulty breathing
- Reduced sperm count (temporary and reversible after stopping the drug)
People with sulfa allergies, liver disease, or blood disorders should avoid this medication unless specifically advised by their doctor.
Patients should seek medical attention immediately for signs of severe rash, persistent fever, sore throat, unusual bruising, or yellowing of the skin or eyes, as these may indicate rare but serious reactions.
Doctors typically order regular blood tests to check liver function, kidney health, and blood cell counts during treatment. These safety checks allow early detection of any potential side effects.
Sulfasalazine dosage
Sulfasalazine is available in tablet form, including delayed-release (enteric-coated) versions to minimize stomach irritation.
For ulcerative colitis, it’s often started at a low dose and gradually increased to manage inflammation effectively while limiting side effects. In rheumatoid arthritis, it is used as part of long-term therapy to help control disease progression.
Monitoring is essential during treatment. Doctors may check:
- Complete blood counts to ensure healthy white and red blood cell levels.
- Liver and kidney function tests at regular intervals.
- Response to therapy, such as reduced joint pain or improved bowel regularity.
Patients are encouraged to stay well hydrated while taking sulfasalazine to prevent kidney-related complications and report any persistent side effects.
Older adults or those with kidney or liver impairment may need closer supervision or dose adjustments.
Does Sulfasalazine have a generic version?
Yes. Sulfasalazine is available in generic form, which is both FDA-approved and widely used. Generic versions are equally safe, effective, and affordable compared to brand-name products.
Common brand names include Azulfidine and Azulfidine EN-Tabs (the enteric-coated formulation). Generic versions are available under the name of sulfasalazine in most pharmacies and hospitals worldwide.
Because the medication has been on the market for decades, access and affordability are generally good, making it an important option in long-term inflammatory disease management.
Conclusion
Sulfasalazine remains a cornerstone therapy for ulcerative colitis and rheumatoid arthritis, thanks to its proven ability to reduce inflammation, control symptoms, and improve long-term outcomes. Its dual action, working locally in the gut and systemically on the immune system, makes it especially valuable for chronic inflammatory conditions.
While side effects and monitoring requirements are important to consider, most patients tolerate the medication well under medical supervision.
When prescribed and monitored by a qualified healthcare provider, sulfasalazine is a safe, effective, and time-tested treatment that can help patients regain comfort, mobility, and control over their condition leading to a better quality of life.
References
- U.S. Food and Drug Administration (FDA). (2023). Sulfasalazine prescribing information. https://www.fda.gov/
- Mayo Clinic. (2023). Sulfasalazine (oral route) description and precautions. https://www.mayoclinic.org/
- O’Dell, J. R., et al. (1996). Treatment of rheumatoid arthritis with methotrexate and sulfasalazine combination therapy. New England Journal of Medicine, 334(20), 1287–1291. https://www.nejm.org/
- MedlinePlus. (2024). Sulfasalazine – Drug information. https://medlineplus.gov/
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- in the treatment of mild to moderate ulcerative colitis, and as adjunctive therapy in severe ulcerative colitis;
- for the prolongation of the remission period between acute attacks of ulcerative colitis;
- in the treatment of patients with rheumatoid arthritis who have responded inadequately to salicylates or other nonsteroidal anti-inflammatory drugs (e.g., an insufficient therapeutic response to, or intolerance of, an adequate trial of full doses of one or more nonsteroidal anti-inflammatory drugs); and
- in the treatment of pediatric patients with polyarticular-course
- van Rossum MAJ, et al. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Arth Rheum 1998;41:808–816.
- Mogadam M, et al. Pregnancy in inflammatory bowel disease: effect of sulfasalazine and corticosteroids on fetal outcome. Gastroenterology 1981;80:726.
- Kaufman DW, editor. Birth defects and drugs during pregnancy. Littleton, MA: Publishing Sciences Group, Inc., 1977:296–313.
- Jarnerot G. Fertility, sterility and pregnancy in chronic inflammatory bowel disease. Scand J Gastroenterol 1982;17:1–4.
- Imundo LF, Jacobs JC. Sulfasalazine therapy for juvenile rheumatoid arthritis. J Rheumatol 1996;23:360–366.
- Hertzberger-ten Cate R, Cats A. Toxicity of sulfasalazine in systemic juvenile chronic arthritis. Clin Exp Rheumatol 1991;9:85–8.
- Farr M, et al. Immunodeficiencies associated with sulphasalazine therapy in inflammatory arthritis. British Jnl Rheum 1991;30:413–417.
- Korelitz B, et al. Desensitization to sulfasalazine in allergic patients with IBD: an important therapeutic modality. Gastroenterology 1982;82:1104.
- Holdworth CG. Sulphasalazine desensitization. Br Med J 1981;282:110.
- Taffet SL, Das KM. Desensitization of patients with inflammatory bowel disease to sulfasalazine. Am J Med 1982;73:520–4.
tablets, USP