Generic Name
Vigabatrin
Brand Names
Vigadrone, Sabril, Vigpoder, Vigafyde
FDA approval date: August 21, 2009
Classification: Anti-epileptic Agent
Form: Tablet, Powder, For, Solution
What is Vigadrone (Vigabatrin)?
Vigabatrin is indicated for the treatment of: Refractory Complex Partial Seizures as adjunctive therapy in patients 2 years of age and older who have responded inadequately to several alternative treatments; vigabatrin is not indicated as a first line agent.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
VIGADRONE (Vigabatrin)
WARNING: PERMANENT VISION LOSS
- VIGADRONE can cause permanent bilateral concentric visual field constriction, including tunnel vision that can result in disability. In some cases, VIGADRONE also can damage the central retina and may decrease visual acuity
- The onset of vision loss from VIGADRONE is unpredictable and can occur within weeks of starting treatment or sooner, or at any time after starting treatment, even after months or years.
- Symptoms of vision loss from VIGADRONE are unlikely to be recognized by patients or caregivers before vision loss is severe. Vision loss of milder severity, while often unrecognized by the patient or caregiver, can still adversely affect function.
- The risk of vision loss increases with increasing dose and cumulative exposure, but there is no dose or exposure known to be free of risk of vision loss.
- Vision assessment is recommended at baseline (no later than 4 weeks after starting VIGADRONE), at least every 3 months during therapy, and about 3 to 6 months after the discontinuation of therapy.
- Once detected, vision loss due to VIGADRONE is not reversible. It is expected that, even with frequent monitoring, some patients will develop severe vision loss.
- Consider drug discontinuation, balancing benefit and risk, if vision loss is documented.
- Risk of new or worsening vision loss continues as long as VIGADRONE is used. It is possible that vision loss can worsen despite discontinuation of VIGADRONE.
- Because of the risk of vision loss, VIGADRONE should be withdrawn from patients with refractory complex partial seizures who fail to show substantial clinical benefit within 3 months of initiation and within 2 to 4 weeks of initiation for patients with infantile spasms, or sooner if treatment failure becomes obvious. Patient response to and continued need for VIGADRONE should be periodically reassessed.
- VIGADRONE should not be used in patients with, or at high risk of, other types of irreversible vision loss unless the benefits of treatment clearly outweigh the risks.
- VIGADRONE should not be used with other drugs associated with serious adverse ophthalmic effects such as retinopathy or glaucoma unless the benefits clearly outweigh the risks.
- Use the lowest dosage and shortest exposure to VIGADRONE consistent with clinical objectives
Because of the risk of permanent vision loss, VIGADRONE is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Vigabatrin REMS Program
1DOSAGE FORMS AND STRENGTHS
VIGADRONE tablets, 500 mg for oral use are white to off-white, oval, film-coated, biconvex tablets, debossed with "ZNV" on one side and scored on the other side.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are described elsewhere in labeling:
- Permanent Vision Loss
- Magnetic Resonance Imaging (MRI) Abnormalities in Infants
- Neurotoxicity
- Suicidal Behavior and Ideation
- Withdrawal of Antiepileptic Drugs (AEDs)
- Anemia
- Somnolence and Fatigue
- Peripheral Neuropathy
- Weight Gain
- Edema
3.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In U.S. and primary non-U.S. clinical studies of 4,079 vigabatrin-treated patients, the most common (≥5%) adverse reactions associated with the use of vigabatrin in combination with other AEDs were headache, somnolence, fatigue, dizziness, convulsion, nasopharyngitis, weight gain, upper respiratory tract infection, visual field defect, depression, tremor, nystagmus, nausea, diarrhea, memory impairment, insomnia, irritability, abnormal coordination, blurred vision, diplopia, vomiting, influenza, pyrexia, and rash.
The adverse reactions most commonly associated with vigabatrin treatment discontinuation in ≥1% of patients were convulsion and depression.
In patients with infantile spasms, the adverse reactions most commonly associated with vigabatrin treatment discontinuation in ≥1% of patients were infections, status epilepticus, developmental coordination disorder, dystonia, hypotonia, hypertonia, weight gain, and insomnia.
3.2Post Marketing Experience
The following adverse reactions have been identified during post approval use of vigabatrin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions are categorized by system organ class.
Birth Defects: Congenital cardiac defects, congenital external ear anomaly, congenital hemangioma, congenital hydronephrosis, congenital male genital malformation, congenital oral malformation, congenital vesicoureteric reflux, dentofacial anomaly, dysmorphism, fetal anticonvulsant syndrome, hamartomas, hip dysplasia, limb malformation, limb reduction defect, low set ears, renal aplasia, retinitis pigmentosa, supernumerary nipple, talipes
Ear Disorders: Deafness
Endocrine Disorders: Delayed puberty
Gastrointestinal Disorders: Gastrointestinal hemorrhage, esophagitis
General Disorders: Developmental delay, facial edema, malignant hyperthermia, multi-organ failure
Hepatobiliary Disorders: Cholestasis
Nervous System Disorders: Dystonia, encephalopathy, hypertonia, hypotonia, muscle spasticity, myoclonus, optic neuritis, dyskinesia
Psychiatric Disorders: Acute psychosis, apathy, delirium, hypomania, neonatal agitation, psychotic disorder
Respiratory Disorders: Laryngeal edema, pulmonary embolism, respiratory failure, stridor
Skin and Subcutaneous Tissue Disorders: Angioedema, maculo-papular rash, pruritus, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), alopecia
4DESCRIPTION
VIGADRONE (vigabatrin, USP) is an oral antiepileptic drug and is available as a white, film- coated 500 mg tablet.
The chemical name of vigabatrin, a racemate consisting of two enantiomers, is (±) 4-amino-5-hexenoic acid. The molecular formula is C
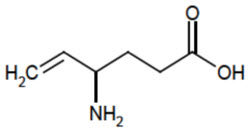
Vigabatrin, USP is a white to off-white powder which is freely soluble in water, slightly soluble in methyl alcohol, very slightly soluble in ethyl alcohol and chloroform, and insoluble in toluene and hexane. The pH of a 1% aqueous solution is about 6.9. The n-octanol/water partition coefficient of vigabatrin is about 0.011 (log
Each VIGADRONE tablet contains 500 mg of vigabatrin. The inactive ingredients are magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate (potato) and isopropyl alcohol. The tablet coating ingredients are hydroxypropyl methylcellulose, polyethylene glycol and titanium dioxide.
Approved dissolution test specifications differ from USP.
5PATIENT COUNSELING INFORMATION
Advise patients and caregivers to read the FDA-approved patient labeling (Medication Guide).
6PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
NDC 0245-6001-11
VIGADRONE™
500 mg
PHARMACIST:
100 Tablets
UPSHER-SMITH