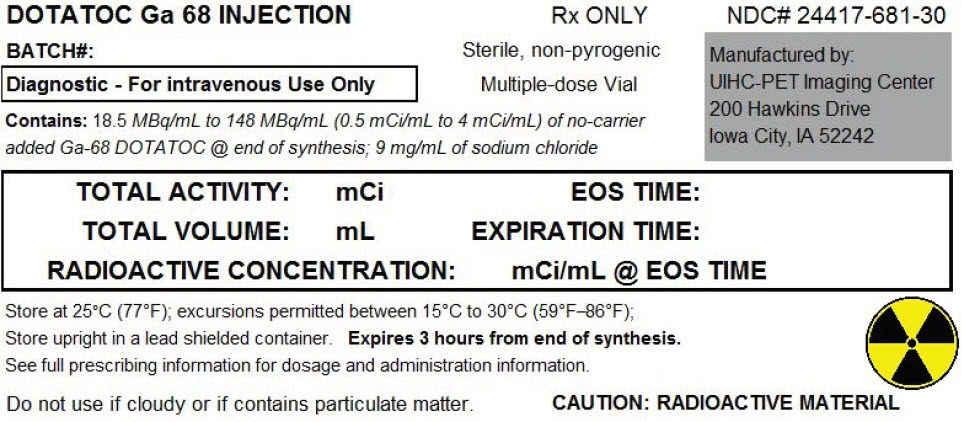
Ga-68-DOTATOC
What is Ga-68-DOTATOC (Edotreotide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of the study is to determine the appropriate pediatric dosage and evaluate the pharmacokinetics (PK) and safety of Lutetium Lu 177 Edotreotide Targeted Radiopharmaceutical Therapy (RPT) as a monotherapy or following standard of care (SoC) in participants ≥2 to \<18 years of age with somatostatin receptor (SSTR)-positive tumors.
Summary: This phase II trial studies how well lutathera works in treating patients with meningioma that cannot be treated with surgery (inoperable) and is growing, spreading, or getting worse (progressive) after external beam radiation therapy. Lutathera is a radioactive drug administered in the vein that is designed to target and kill tumor cells. The goal of this study is to determine whether this drug i...
Summary: Participants in this study will be patients diagnosed with or suspected to have a thyroid nodule or thyroid cancer. The main purpose of this study is to further understand the methods for the diagnosis and treatment of thyroid nodules and thyroid cancer. Many of the test performed are in the context of standard medical care that is offered to all patients with thyroid nodules or thyroid cancer. Ot...
Related Latest Advances
Brand Information
- Hypersensitivity reactions