Fareston
What is Fareston (Toremifene)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The aim of this prospective study is to investigate quality of life and oncologic outcomes in low-risk elderly breast cancer patients randomized to adjuvant therapy with accelerated partial breast irradiation (APBI) alone or endocrine therapy alone after lumpectomy. The study population will include women age 65 years and older with low-risk tumor characteristics (tumor size \<2cm, grade 1-2, node...
Summary: This is an open label, phase II study evaluating the efficacy and safety of trastuzumab combined with oral chemotherapy or endocrine therapy in patients with HER-2 positive stage I breast cancer.
Summary: This is a prospective, randomized, open-label phase III clinical study on the efficacy and safety of fluzoparib combined with adjuvant endocrine therapy versus adjuvant endocrine therapy for HR+/HER2- SNF3-subtype early breast cancer.
Related Latest Advances
Brand Information
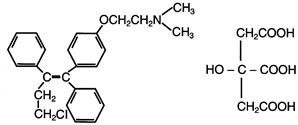
Excursions permitted to 15-30°C (59-86°F)
[See USP Controlled Room Temperature.]
Protect from heat and light.