Generic Name
Beclomethasone Dipropionate
Brand Names
Qvar Redihaler, QNASL
FDA approval date: November 01, 2017
Classification: Corticosteroid
Form: Aerosol
What is Qvar Redihaler (Beclomethasone Dipropionate)?
QNASL Nasal Aerosol is a corticosteroid indicated for the treatment of nasal symptoms associated with seasonal and perennial allergic rhinitis in patients 4 years of age and older.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
QVAR REDIHALER (Beclomethasone Dipropionate HFA)
1INDICATIONS AND USAGE
QVAR REDIHALER is indicated in the maintenance treatment of asthma as prophylactic therapy in adults and pediatric patients 4 years of age and older.
Limitations of Use:
QVAR REDIHALER is not indicated for the relief of acute bronchospasm.
2DOSAGE FORMS AND STRENGTHS
Inhalation aerosol: a pressurized, breath‑actuated, metered-dose aerosol with a dose counter in 2 strengths
- 40 mcg in an aluminum canister contained within a beige plastic actuator and a hinged white cap
- 80 mcg in an aluminum canister contained within a maroon plastic actuator and a hinged white cap
3CONTRAINDICATIONS
QVAR REDIHALER is contraindicated in:
- the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required
- in patients with known hypersensitivity to beclomethasone dipropionate or any of the ingredients in QVAR REDIHALER
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Oropharyngeal candidiasis
- Immunosuppression and risk of infections
- Hypercorticism and adrenal suppression
- Reduction in bone mineral density
- Growth effects
- Glaucoma and cataracts
4.1Clinical Trials Experience
A total of 1858 subjects participated in the QVAR REDIHALER clinical development program. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults and Adolescent Patients 12 years of Age and Older: The adverse reaction information presented in Table 1 is derived from 3 double-blind, placebo-controlled clinical trials in which 1230 patients (751 female and 479 male adults previously treated with as‑needed bronchodilators and/or inhaled corticosteroids) were treated with QVAR REDIHALER (doses of 40, 80, 160, or 320 mcg twice daily) or QVAR (beclomethasone dipropionate HFA) Inhalation Aerosol (QVAR MDI; doses of 160 or 320 mcg twice daily) or placebo. In considering these data, difference in average duration of exposure and clinical trial design should be taken into account.
Table 1: Adverse Reactions Experienced by at Least 3% of Adult and Adolescent Patients in the QVAR REDIHALER or QVAR MDI Groups and Greater Than Placebo by Treatment and Daily Dose
*QVAR MDI=QVAR Inhalation Aerosol
Other adverse reactions that occurred in clinical trials using QVAR REDIHALER with an incidence of 1% to 3% and which occurred at a greater incidence than placebo were back pain, headache, pain, nausea and cough.
Pediatric Patients 4 to 11 Years of Age: The adverse reaction information presented in Table 2 concerning QVAR REDIHALER and QVAR MDI is derived from one 12‑week placebo-controlled study in pediatric patients 4 to 11 years of age with persistent asthma.
Table 2: Adverse Reactions Experienced by at Least 3% of Patients 4 to 11 Years of Age in the QVAR REDIHALER or QVAR MDI Groups and Greater Than Placebo by Treatment and Daily Dose
*QVAR MDI=QVAR Inhalation Aerosol
Other adverse reactions that occurred in clinical trials using QVAR REDIHALER with an incidence of 1% to 3% and which occurred at a greater incidence than placebo were influenza, gastroenteritis viral, ear infection, oral candidiasis, diarrhea, and myalgia.
4.2Postmarketing Experience
In addition to the adverse reactions reported from clinical trials with QVAR REDIHALER, the following adverse reactions have been identified during post-approval use of QVAR MDI and other inhaled corticosteroids. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Local Effects: Localized infections with Candida albicans have occurred in patients treated with beclomethasone dipropionate or other orally inhaled corticosteroids [see Warnings and Precautions (.
Psychiatric and Behavioral Changes: Aggression, depression, sleep disorders, psychomotor hyperactivity, and suicidal ideation have been reported (primarily in children).
Eye Disorders: Blurred vision, central serous chorioretinopathy (CSC).
5DESCRIPTION
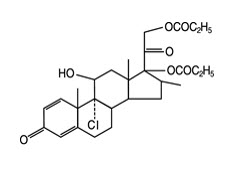
The active component of QVAR REDIHALER 40 mcg Inhalation Aerosol and QVAR REDIHALER 80 mcg Inhalation Aerosol is beclomethasone dipropionate, USP, a corticosteroid having the chemical name 9-chloro-11ß,17,21-trihydroxy-16ß-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate. Beclomethasone dipropionate is a diester of beclomethasone, a synthetic corticosteroid chemically related to dexamethasone. Beclomethasone differs from dexamethasone in having a chlorine at the 9‑alpha carbon in place of a fluorine, and in having a 16‑beta-methyl group instead of a 16‑alpha-methyl group. Beclomethasone dipropionate is a white to creamy white, odorless powder with a molecular formula of C
QVAR REDIHALER is a pressurized, breath‑actuated, metered‑dose aerosol with a dose counter intended for oral inhalation only. Each unit consists of a sealed breath‑actuated inhaler device enclosing a canister containing a solution of beclomethasone dipropionate in propellant HFA‑134a (1,1,1,2 tetrafluoroethane) and ethanol (0.85 g). QVAR REDIHALER 40 mcg delivers 40 mcg of beclomethasone dipropionate from the actuator mouthpiece and 50 mcg from the canister valve. QVAR REDIHALER 80 mcg delivers 80 mcg of beclomethasone dipropionate from the actuator mouthpiece and 100 mcg from the canister valve. Both products deliver 50 microliters (59 milligrams) of solution formulation as an aerosol from the canister valve with each actuation. The 40‑mcg canisters and the 80‑mcg canisters provide 120 inhalations each. Since the QVAR REDIHALER canister is fitted with a primeless valve, no priming actuations are required before use. For both products, an actuation was always triggered by a 20 L/min inspiratory flow rate.
6CLINICAL STUDIES
The safety and efficacy of QVAR REDIHALER were evaluated in 1,858 patients with asthma. The development program included 2 confirmatory trials of 12 weeks duration and 1 confirmatory trial of 6 weeks duration in patients 12 years of age and older, and 1 confirmatory trial of 12 weeks duration in patients 4 to 11 years of age. The efficacy of QVAR REDIHALER is based primarily on the confirmatory trials described below.
6.1Trials in the Maintenance Treatment of Asthma
Adult and Adolescent Patients 12 Years of Age and Older
Two confirmatory clinical trials were conducted comparing QVAR REDIHALER with placebo in adult and adolescent patients with persistent asthma (Trial 1 and Trial 2).
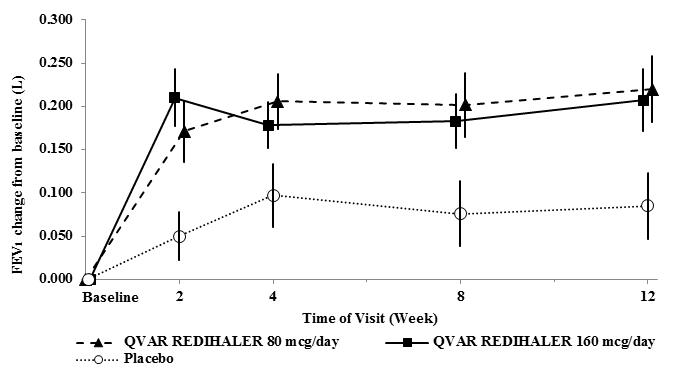
Trial 1 (NCT02040779): This randomized, double-blind, parallel-group, placebo-controlled, 12-week, efficacy and safety trial compared QVAR REDIHALER 40 and 80 mcg given as 1 inhalation twice daily with placebo in adult and adolescent patients with persistent symptomatic asthma despite low-dose inhaled corticosteroid or non-corticosteroid asthma therapy. Patients aged 12 years and older who met the entry criteria including FEV
In addition, the mean change from baseline is displayed in Figure 1. Both doses of QVAR REDIHALER were effective in improving asthma control with significantly greater improvements in FEV
Figure 1: A 12‑Week Clinical Trial in Patients with Asthma: Mean Change in FEV
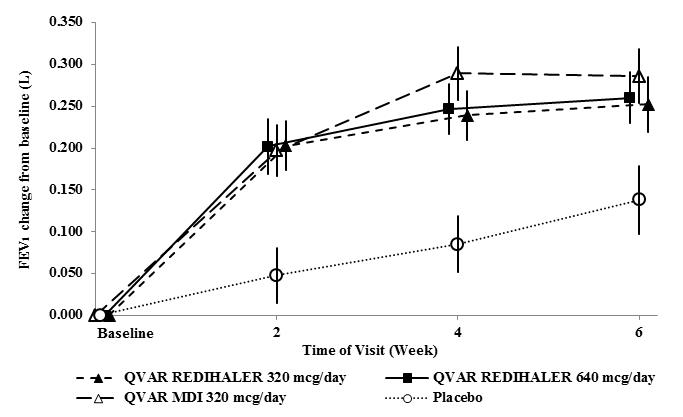
Trial 2 (NCT02513160): This randomized, double-blind, parallel-group, placebo-controlled, 6-week, efficacy and safety trial compared QVAR REDIHALER 40 and 80 mcg given as 4 inhalations twice daily and placebo in adult and adolescent patients with persistent symptomatic asthma despite treatment with non-corticosteroid, inhaled corticosteroids (with or without a long acting beta agonist [LABA]), or combination asthma therapy. The study also included a reference treatment group, QVAR
Figure 2: A 6‑Week Dose Response Clinical Trial in Patients with Inhaled Corticosteroid-Dependent Asthma: Mean Change in FEV
Side-by-side comparison of the primary analysis of standardized baseline-adjusted trough morning FEV
Table 3: Primary Analysis of Standardized Baseline-Adjusted Trough Morning FEVfrom Time Zero to the End of the Treatment Period 12-week Study and 6-week Dose Response Study
*QVAR MDI=QVAR Inhalation Aerosol
Pediatric Patients 4 to 11 Years of Age
This randomized, double-blind, parallel-group, placebo controlled, 12-week, global efficacy and safety trial (NCT02040766) compared QVAR REDIHALER 40 or 80 mcg, QVAR MDI 40 or 80 mcg or placebo given as 1 inhalation twice daily in pediatric patients aged 4 through 11 years old with persistent symptomatic asthma despite treatment with non-corticosteroid or low dose inhaled corticosteroid (with or without a long acting beta agonist [LABA]). Patients aged 4 to 5 years who were technically unable to complete spirometry participated in the safety population. Patients who met the entry criteria including FEV
7HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-5032
NDC: 50090-5032-0 1 AEROSOL, METERED in a INHALER / 1 in a CARTON
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA‑Approved Patient Labeling (Patient Information and Instructions for Use).
Patients should be given the following information:
Oropharyngeal Candidiasis
Inform patients that localized infections with
Status Asthmaticus and Acute Asthma Symptoms
Inform patients that QVAR REDIHALER is not a bronchodilator and is not intended for use as rescue medicine for acute asthma exacerbations. Advise patients to treat acute asthma symptoms with an inhaled, short‑acting beta
Immunosuppression and Risk of Infections
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Hypercorticism and Adrenal Suppression
Advise patients that QVAR REDIHALER may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, instruct patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to QVAR REDIHALER.
Immediate Hypersensitivity Reactions
Advise patients that immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, and hypotension), including anaphylaxis, may occur after administration of QVAR REDIHALER. Patients should discontinue QVAR REDIHALER if such reactions occur and contact their healthcare provider or get emergency medical help.
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk.
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including QVAR REDIHALER, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of adolescents taking corticosteroids by any route.
Ocular Effects
Long-term use of inhaled corticosteroids may increase the risk of some eye problems (cataracts, glaucoma or blurred vision); consider regular eye examinations.
Pregnancy
Inform patients who are pregnant or nursing that they should contact their physician about the use of QVAR REDIHALER.
Use Daily for Best Effect
Patients should use QVAR REDIHALER at regular intervals as directed. The daily dosage of QVAR REDIHALER should not exceed 8 inhalations per day. Advise patients, if they miss a dose, to take their next dose at the same time they normally do. Individual patients will experience a variable time to onset and degree of symptom relief and the full benefit may not be achieved until treatment has been administered for 1 to 2 weeks or longer. Patients should not increase the prescribed dosage but should contact their physicians if symptoms do not improve or if the condition worsens. Instruct patients to not stop use of QVAR REDIHALER abruptly. Patients should contact their physicians immediately if they discontinue use of QVAR REDIHALER.
Caring for and Storing the Inhaler
For normal hygiene, the mouthpiece of QVAR REDIHALER should be cleaned weekly with a clean, dry tissue or cloth. Never wash or put any part of QVAR REDIHALER in water. Patient should replace QVAR REDIHALER if washed or placed in water.
Instruct patients to store the inhaler at room temperature and to avoid exposure to extreme heat and cold.
Inform patients that shaking the inhaler prior to use is not necessary. Instruct patients not to shake the inhaler with the cap open to avoid possible actuation of the device.
Instruct patients to never take QVAR REDIHALER apart.
Inform patients that QVAR REDIHALER has a dose counter attached to the actuator at the rear of the mouth piece. When the patient receives the inhaler, the number 120 will be displayed. The dose counter will count down each time a spray is released. The dose-counter window displays the number of sprays left in the inhaler in units of two (e.g., 120, 118, 116, etc). When the counter displays 20, the color of the numbers will change to red to remind the patient to contact their pharmacist for a refill of medication or consult their healthcare provider for a prescription refill. When the dose counter reaches 0, the background will change to solid red. Inform patients to discard QVAR REDIHALER when the dose counter displays 0 or after the expiration date on the product, whichever comes first.
Distributed by:
Teva Pharmaceuticals USA
Parsippany, NJ 07054
© 2021 Teva Respiratory, LLC
U.S. Patent 7,637,260; 8,132,712; 8,931,476
QVARH-003
9PATIENT INFORMATION
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 01/2021
10INSTRUCTIONS FOR USE
Instructions for Use
QVAR REDIHALER)
(beclomethasone dipropionate HFA)
inhalation aerosol
Your QVAR REDIHALER Inhaler
Overview
When you are ready to use your QVAR REDIHALER for the first time, remove the inhaler from the carton.
Important information:
- There is no button. You must close the white cap to prepare the inhaler with medicine
- Do not shake. This breath-actuated device does not need to be shaken. This is not a press-and-breathe inhaler.
- Do not prime QVAR REDIHALER. The inhaler does not need to be primed.
- Do not use a spacer or volume holding chamber with QVAR REDIHALER.
- Always use the inhaler in the upright position (with the mouthpiece down).
- After the inhaler is prepared, it will deliver 1 inhalation of medicine when you breathe in (inhale) through the mouthpiece. Your dose might require more than 1 inhalation.
- Do not open the white cap or leave it open unless you are ready for your next inhalation. If the cap has been opened for more than 2 minutes or left in the open position, you will need to close the white cap before use.
- Do not breathe out or blow into any part of the inhaler. Breathing out or blowing into the inhaler can damage it.
- Do not suddenly stop using your QVAR REDIHALER. Contact your healthcare provider immediately if you stop using your QVAR REDIHALER.
There are 2 main parts of your QVAR REDIHALER including:
- the inhaler body with the mouthpiece.
- the white cap that covers the mouthpiece of the inhaler.
Figure A
About the Dose Counter
There is a dose counter in the back of the inhaler with a viewing window that shows you how many inhalations of medicine you have left.
- Your QVAR REDIHALER contains 120 inhalations.
- The counter on the back of your inhaler shows how many inhalations you have left. When there are 20 inhalations left, the numbers in the dose counter will change to red and you should refill your prescription or ask your healthcare provider for another prescription.
- When the dose counter shows ‘0’, the background will turn solid red and your inhaler is empty. You should stop using the inhaler and throw it away.
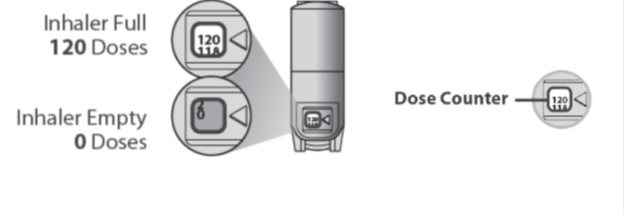
Figure B
Important:
- The white cap must be closed to prepare the inhaler before each inhalation or you will not receive your medicine. See Figure C.
- If the white cap is open, close the white cap to prepare your inhaler and look at the dose counter window to make sure that your inhaler is not empty.
- Do not open the cap until you are ready to take your inhalation.
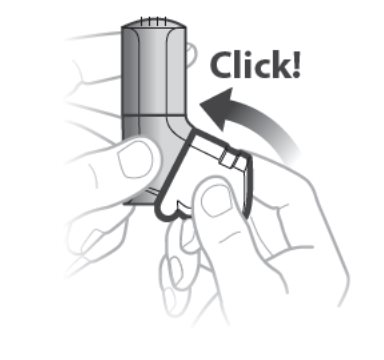
Figure C
Using your QVAR REDIHALER:
Step 1. Open the white cap
Figure D
Remember:
- Do not open the cap until you are ready to take your inhalation.
- Never breathe out or blow into the inhaler. Breathing out or blowing into the inhaler can damage it.
Step 2. Inhale 1 Time
Figure E
Remember:
- Hold the inhaler upright as you take your inhalation.
Step 3. Close the white cap
Figure F
If your healthcare provider has told you to take more than 1 inhalation per dose, make sure the white cap is closed and repeat Step 1 to Step 3.
After taking your prescribed number of inhalations, rinse your mouth with water
How to store your QVAR REDIHALER
- Store QVAR REDIHALER at room temperature between 68ºF to 77ºF (20ºC to 25ºC). Avoid exposure to extreme heat or cold.
- Keep the white cap on the inhaler closed during storage.
- Keep your QVAR REDIHALER inhaler dry and clean at all times.
- If you drop your QVAR REDIHALER, inspect it for damage before use. If the QVAR REDIHALER is damaged,
- Do not use or store your QVAR REDIHALER near heat or open flame. Exposure to temperatures above 120ºF (49ºC) may cause the canister to burst.
- Do not throw QVAR REDIHALER into fire or an incinerator.
- Throw away QVAR REDIHALER when the dose counter displays ‘0,’ or after the expiration date on the package, whichever comes first.
- Keep your QVAR REDIHALER and all medicines out of the reach of children.
Cleaning your QVAR REDIHALER
- Do not wash or put any part of your QVAR REDIHALER in water.
- Clean the mouthpiece of your QVAR REDIHALER weekly with a clean, dry tissue or cloth.
Support
- If you have any questions about QVAR REDIHALER or how to use your inhaler, go to www.QVAR.com or call 1-888-483-8279.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
©2021Teva Respiratory, LLC. All rights reserved.
QVARHIFU-003
Rev. 01/2021
11Beclomethasone Dipropionate HFA
