Generic Name
Tacrolimus
Brand Names
Envarsus, Prograf, Tacrus, Astagraf
FDA approval date: April 08, 1994
Classification: Calcineurin Inhibitor Immunosuppressant
Form: Ointment, Injection, Tablet, Granule, Capsule
What is Envarsus (Tacrolimus)?
Tacrolimus Capsules USP is a calcineurin-inhibitor immuno-suppressant indicated for the prophylaxis of organ rejection in adult and pediatric patients receiving allogeneic liver, kidney, heart or lung transplants, in combination with other immunosuppressants.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Envarsus (Tacrolimus)
WARNING: MALIGNANCIES AND SERIOUS INFECTIONS
Increased risk for developing serious infections and malignancies with ENVARSUS XR or other immunosuppressants that may lead to hospitalization or death
1DOSAGE FORMS AND STRENGTHS
Oval, white to off-white uncoated extended-release tablets debossed with “TCS” on one side:
- 0.75 mg extended-release tablet: debossed with “0.75” on the other side.
- 1 mg extended-release tablet: debossed with “1” on the other side.
- 4 mg extended-release tablet: debossed with “4” on the other side.
2CONTRAINDICATIONS
ENVARSUS XR is contraindicated in patients with known hypersensitivity to tacrolimus or to any of the ingredients in ENVARSUS XR.
3ADVERSE REACTIONS
The following clinically significant adverse drug reactions are discussed in greater detail in other sections of the labeling:
- Lymphoma and Other Malignancies
- Serious Infections
- New Onset Diabetes after Transplant
- Nephrotoxicity due to ENVARSUS XR and Drug Interactions
- Neurotoxicity
- Hyperkalemia
- Hypertension
- QT Prolongation
- Pure Red Cell Aplasia
- Thrombotic Microangiopathy, Including Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura
3.1Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinicalstudies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the ratesobserved in practice. In addition, the clinical studies were not designed to establish comparative differences across studyarms with regards to the adverse reactions discussed below.
Study 1- Phase 3 Clinical Study in De Novo Kidney Transplant Recipients
Study 1 (NCT 01187953), was a Phase 3 randomized study in de novo kidney transplant patients that were treated withENVARSUS XR (N=268) or tacrolimus [immediate-release] capsules (N=275) and concomitant immunosuppressants in adouble-blind, randomized, multinational study
Infections
The overall incidence of infections, serious infections, and infections with identified etiology reported in de novo kidneytransplant recipients treated with ENVARSUS XR or tacrolimus [immediate-release] capsules in Study 1 are shown in
New Onset Diabetes After Transplantation
New onset diabetes after transplantation (NODAT) was defined by the composite occurrence of fasting plasma glucosevalues ≥126 mg/dL, 2-hour post-prandial plasma glucose of at least 200 mg/dL (in oral glucose tolerance test) on two ormore consecutive occasions post-baseline, insulin requirement for ≥31 days, an oral hypoglycemic agent use ≥31 days, orHbA
Common Adverse Reactions
The incidence of adverse reactions that occurred in ≥10% of ENVARSUS XR-treated patients compared to tacrolimus[immediate-release] capsules through one year of treatment in Study 1 is shown by treatment group in
Study 2- Phase 2 Clinical Study in De Novo Kidney Transplant Recipients
Study 2 (NCT00765661) was an open-label Phase 2 study conducted in de novo kidney transplant patients randomized toonce daily ENVARSUS XR (N=32) or twice daily tacrolimus [immediate-release] capsules (N=31). The study wasconducted in the US and patients received an organ from a deceased or living donor. Pharmacokinetics were evaluatedduring the first 2 weeks with an additional 50-week treatment and follow-up to evaluate safety and efficacy
The starting dosage was 0.14 mg/kg/day (given once daily) for ENVARSUS XR and 0.2 mg/kg/day (given twice daily)for tacrolimus [immediate-release] capsules. On Day 2 predose, the proportion of patients in the ENVARSUS XR groupwith tacrolimus trough concentration that were within, above, and below 6 to 11 ng/mL was 53%, 11%, and 37%,respectively. The starting dose of 0.14 mg/kg/day in Study 2 formed the basis of dosing recommendations in de novokidney transplant patients.
There were no deaths or graft failures in Study 2. Two patients in each arm discontinued due to adverse events. The mostcommon adverse reactions included infections and cardiovascular events, and were generally similar to those reported inStudy 1.
Study 3- Phase 3 Clinical Studies in Stable Kidney Transplant Recipients Converted from Tacrolimus Capsules
In Study 3 (NCT00817206) stable kidney transplant patients were treated with ENVARSUS XR (N=162) or tacrolimus[immediate-release] capsules (N=162) and concomitant immunosuppressants in an open-label, randomized, multinationalstudy
Infections
The overall incidence of infections, serious infections, and infections with identified etiology reported in stable kidneytransplant recipients treated with ENVARSUS XR or tacrolimus capsules are shown in
New Onset Diabetes After Transplantation
New onset diabetes after transplantation (NODAT) was defined by the composite occurrence of fasting plasma glucosevalues ≥126 mg/dL, 2-hour postprandial plasma glucose of at least 200 mg/dL (in oral glucose tolerance test) on 2 or moreconsecutive occasions post-baseline, insulin requirement for ≥31 days, an oral hypoglycemic agent use ≥31 days, orHbA1c ≥6.5% (at least 3 months after randomization) among kidney transplant patients with no medical history ofdiabetes. The incidence of NODAT for the stable kidney transplant study through one year post-transplant is summarizedin
Common Adverse Reactions
In Study 3, the most common (≥10%) adverse reactions observed with Envarsus XR were diarrhea (14%), and bloodcreatinine increased (12%).
3.2Postmarketing Experience
The following adverse reactions have been reported from marketing experience with tacrolimus in the U.S. and outsidethe U.S. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible toreliably estimate their frequency or establish a causal relationship to drug exposure. The following reactions have beenincluded due to either their seriousness, frequency of reporting or strength of causal connection to ENVARSUS XR:
- Blood and Lymphatic System Disorders: Agranulocytosis, decreased blood fibrinogen, disseminated intravascular coagulation, hemolytic anemia, hemolytic uremic syndrome, leukopenia, febrile neutropenia, pancytopenia, prolonged activated partial thromboplastin time, pure red cell aplasia
- Cardiac Disorders: Atrial fibrillation, atrial flutter, cardiac arrhythmia, cardiac arrest, electrocardiogram T wave abnormal, flushing, myocardial hypertrophy, myocardial infarction, myocardial ischaemia, pericardial effusion, QT prolongation, supraventricular extrasystoles, supraventricular tachycardia,
- Ear Disorders: Hearing loss including deafness
- Eye Disorders: Blindness, optic neuropathy, photophobia, optic atrophy
- Gastrointestinal Disorders: Abdominal pain, colitis, dysphagia, gastrointestinal perforation, impaired gastric emptying, intestinal obstruction, mouth ulceration, peritonitis, stomach ulcer
- Hepatobiliary Disorders: Bile duct stenosis, cholangitis, cirrhosis, fatty liver, hepatic cytolysis, hepatic failure, hepatic necrosis, hepatic steatosis, jaundice, hemorrhagic pancreatitis, necrotizing pancreatitis, venoocclusive liver disease, hepatitis (acute and chronic)
- Hypersensitivity Reactions: Hypersensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria
- Immune System Disorders: Graft versus host disease (acute and chronic)
- Metabolism and Nutrition Disorders: Glycosuria, increased amylase, pancreatitis
- Musculoskeletal and Connective Tissue Disorders: Myalgia, polyarthritis, rhabdomyolysis
- Neoplasms: Lymphoma including EBV-associated lymphoproliferative disorder, PTLD
- Nervous System Disorders: Carpal tunnel syndrome, cerebral infarction, coma, dysarthria, flaccid paralysis, hemiparesis, mental disorder, mutism, nerve compression, posterior reversible encephalopathy syndrome (PRES)
- Renal and Urinary Disorder: Acute renal failure, hemorrhagic cystitis, hemolytic uremic syndrome, micturition disorder
- Respiratory, Thoracic and Mediastinal Disorders: Acute respiratory distress syndrome, interstitial lung disease, lunginfiltration, pulmonary embolism, pulmonary hypertension, respiratory distress, respiratory failure
- Skin and Subcutaneous Tissue Disorders: Hyperpigmentation, photosensitivity, pruritus, rash
4OVERDOSAGE
Postmarketing cases of overdose with tacrolimus have been reported. Overdosage adverse reactions included:
- nervous system disorders (tremor, headache, confusional state, balance disorders, encephalopathy, lethargy andsomnolence)
- gastrointestinal disturbances (nausea, vomiting, and diarrhea)
- abnormal renal function (increased blood urea nitrogen and elevated serum creatinine)
- urticaria
- hypertension
- peripheral edema, and
- infections (one fatal postmarketing case of bilateral pneumopathy and CMV infection was attributed totacrolimus extended-release capsules overdose).
Based on the poor aqueous solubility and extensive erythrocyte and plasma protein binding, it is anticipated thattacrolimus is not dialyzable to any significant extent; there is no experience with charcoal hemoperfusion. The oral use ofactivated charcoal has been reported in treating acute overdoses, but experience has not been sufficient to warrantrecommending its use. General supportive measures and treatment of specific symptoms should be followed in all cases ofoverdosage.
5DESCRIPTION
Tacrolimus is the active ingredient in ENVARSUS XR. Tacrolimus is a calcineurin-inhibitor immunosuppressant produced by
The chemical structure of tacrolimus is:
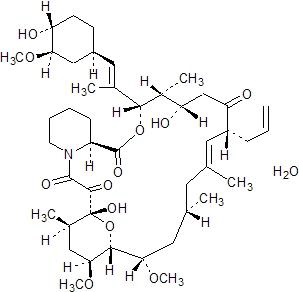
Tacrolimus has an empirical formula of C
ENVARSUS XR is available for oral administration as extended-release tablets containing the equivalent of 0.75 mg,1 mg, or 4 mg of anhydrous tacrolimus USP. Inactive ingredients include hypromellose USP, lactose monohydrate NF,polyethylene glycol NF, poloxamer NF, magnesium stearate NF, tartaric acid NF, butylated hydroxytoluene NF, anddimethicone NF.
6HOW SUPPLIED/STORAGE AND HANDLING
ENVARSUS XR is supplied in round HDPE bottles with twist-off caps (see
Store and Dispense
Store at 25 °C (77 °F); excursions permitted to 15 °C to 30 °C (59 °F to 86 °F) [see USP Controlled Room Temperature].
Store at 25 °C (77 °F); excursions permitted to 15 °C to 30 °C (59 °F to 86 °F) [see USP Controlled Room Temperature].
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
7.1Administration
Advise patients to:
- Inspect their ENVARSUS XR medicine when they receive a new prescription and before taking it. If the appearanceof the tablet is not the same as usual, or if dosage instructions have changed, advise patients to contact theirhealthcare provider as soon as possible to make sure that you have the right medicine. Other tacrolimus productscannot be substituted for ENVARSUS XR
- Take once-daily ENVARSUS XR at the same time every day (preferably in the morning) on an empty stomach. atleast 1 hour before or at least 2 hours after a meal to ensure consistent and maximum possible drug concentrations inthe blood.
- Swallow tablet whole with liquid, preferably water. Do not chew, divide or crush tablet.
- Avoid alcoholic beverages, grapefruit, and grapefruit juice while on ENVARSUS XR
- Take a missed dose as soon as possible but not more than 15 hours after the scheduled time (i.e., for a missed 8 AMdose, take it no later than 10 PM). Beyond the 15-hour timeframe, instruct the patient to wait until the usualscheduled time the following morning to take the next regularly scheduled dose. Do not take two doses at the sametime
7.2Development of Lymphoma and Other Malignancies
Inform patients that they are at an increased risk of developing lymphomas and other malignancies, particularly of the skin, due to immunosuppression. Advise patients to limit exposure to sunlight and ultraviolet (UV) light by wearing protective clothing and use a sunscreen with a high protection factor
7.3Increased Risk of Infection
Inform patients that they are at an increased risk of developing a variety of infections, including opportunistic infections,due to immunosuppression and to contact their physician if they develop any symptoms of infection such as fever, sweatsor chills, cough or flu-like symptoms, muscle aches, or warm, red, painful areas on the skin
7.4New Onset Diabetes After Transplant
Inform patients that ENVARSUS XR can cause diabetes mellitus and should be advised to contact their physician if theydevelop frequent urination, increased thirst or hunger
7.5Nephrotoxicity
Inform patients that ENVARSUS XR can have toxic effects on the kidney that should be monitored. Advise patients toattend all visits and complete all blood tests ordered by their medical team
7.6Neurotoxicity
Inform patients that they are at risk of developing adverse neurologic effects including seizure, altered mental status, andtremor. Advise patients to contact their physician should they develop vision changes, delirium, or tremors
7.7Hyperkalemia
Inform patients that ENVARSUS XR can cause hyperkalemia. Monitoring of potassium levels may be necessary,especially with concomitant use of other drugs known to cause hyperkalemia
7.8Hypertension
Inform patients that ENVARSUS XR can cause high blood pressure which may require treatment with anti-hypertensivetherapy. Advise patients to monitor their blood pressure
7.9Thrombotic Microangiopathy
Inform patients that ENVARSUS XR can cause blood clotting problems. The risk of this occurring increases when patients take ENVARSUS XR and sirolimus or everolimus concomitantly, or when patients develop certain infections. Advise them to seek medical attention promptly if they develop fever, petequiae or bruises, fatigue, confusion, jaundice, oliguria
7.10Drug Interactions
Instruct patients to tell their healthcare providers when they start or stop taking any concomitant medications, includingprescription and non-prescription medicines, natural or herbal remedies, dietary supplements and vitamins. Somemedications could alter tacrolimus concentrations in the blood and thus may require the adjustment of the dosage ofENVARSUS XR. Advise patients to avoid grapefruit, grapefruit juice and alcoholic beverages
7.11Pregnancy, Lactation and Infertility
Inform women of childbearing potential that ENVARSUS XR can harm the fetus. Instruct male and female patients todiscuss with their healthcare provider family planning options including appropriate contraception. Also discuss withpregnant patients the risks and benefits of breastfeeding their infant
Encourage female transplant patients who become pregnant and male patients who have fathered a pregnancy, exposed toimmunosuppressants including tacrolimus, to enroll in the voluntary Transplantation Pregnancy Registry International. Toenroll or register, patients can call the toll-free number 1-877-955-6877 or https://www.transplantpregnancyregistry.org.
Based on animal studies, ENVARSUS XR may affect fertility in males and females
7.12Immunizations
Inform patients that ENVARSUS XR can interfere with the usual response to immunizations and that they should avoidlive vaccines
Product of Germany
Manufactured by:
Manufactured for: