Vyepti
What is Vyepti (Eptinezumab-Jjmr)?
For people who live with chronic migraines, even simple daily activities can feel impossible. The unpredictable pain, light sensitivity, and nausea can interfere with work, relationships, and mental well-being. Vyepti (eptinezumab-jjmr) offers new hope for those who have tried traditional treatments without success. As one of the newest preventive therapies for migraine, it helps reduce the number and severity of migraine attacks, allowing many patients to reclaim control over their lives.
Vyepti belongs to a class of drugs known as CGRP monoclonal antibodies, which represent a modern, targeted approach to migraine prevention. Approved by the U.S. Food and Drug Administration (FDA) in 2020, it’s administered through an intravenous (IV) infusion rather than a pill or injection at home. This innovative design allows for rapid delivery and sustained effectiveness over several months.
What does Vyepti do?
Vyepti is prescribed to prevent migraine headaches in adults, it is not intended to treat a migraine once it starts, but rather to stop attacks from occurring as often.
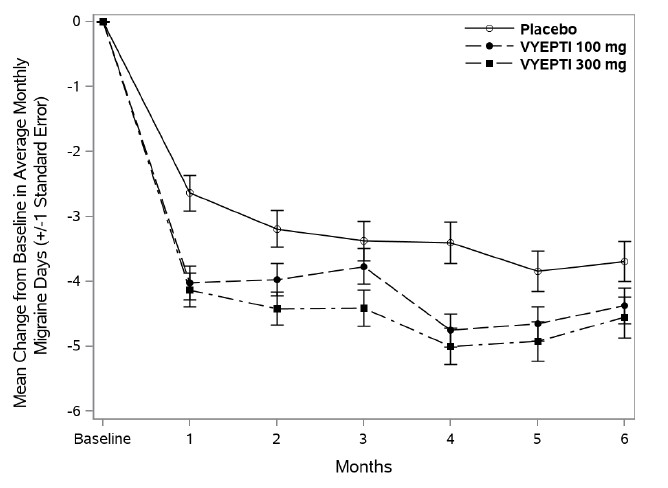
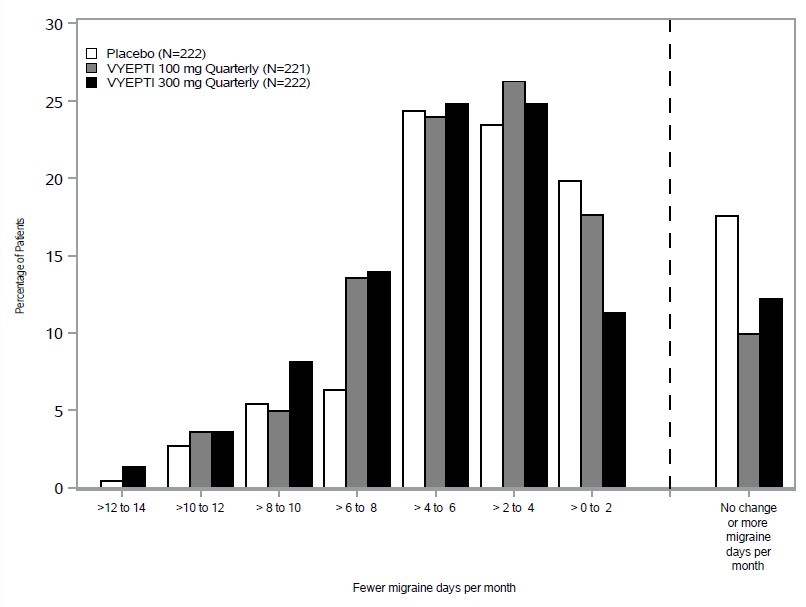
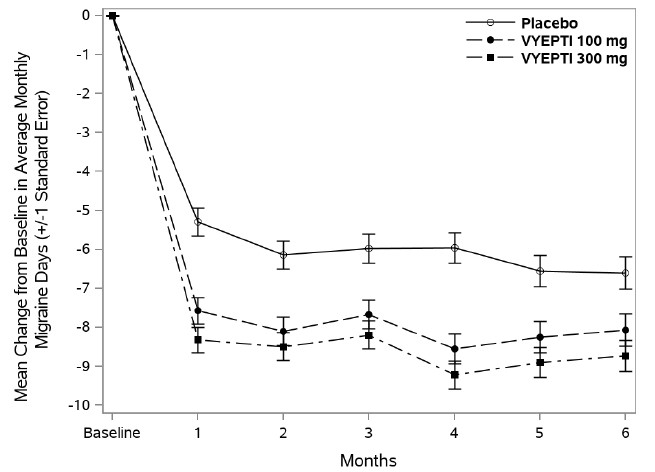
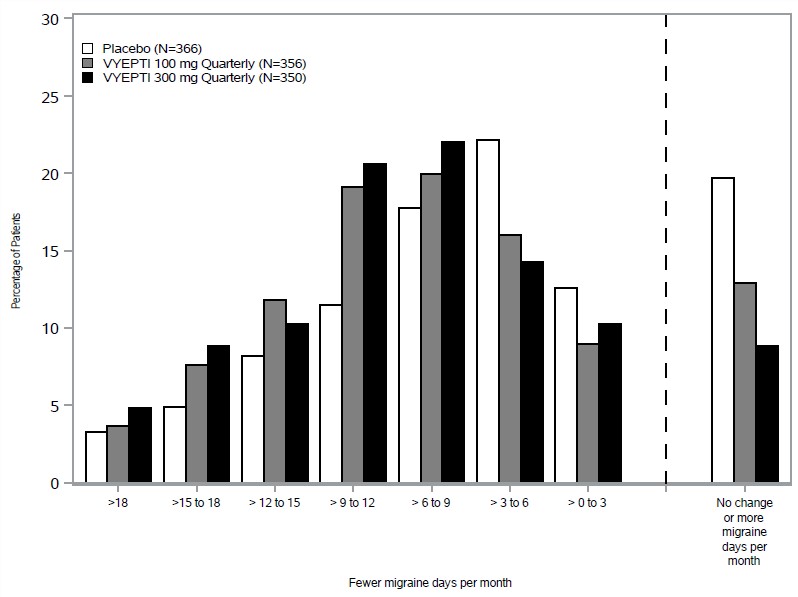
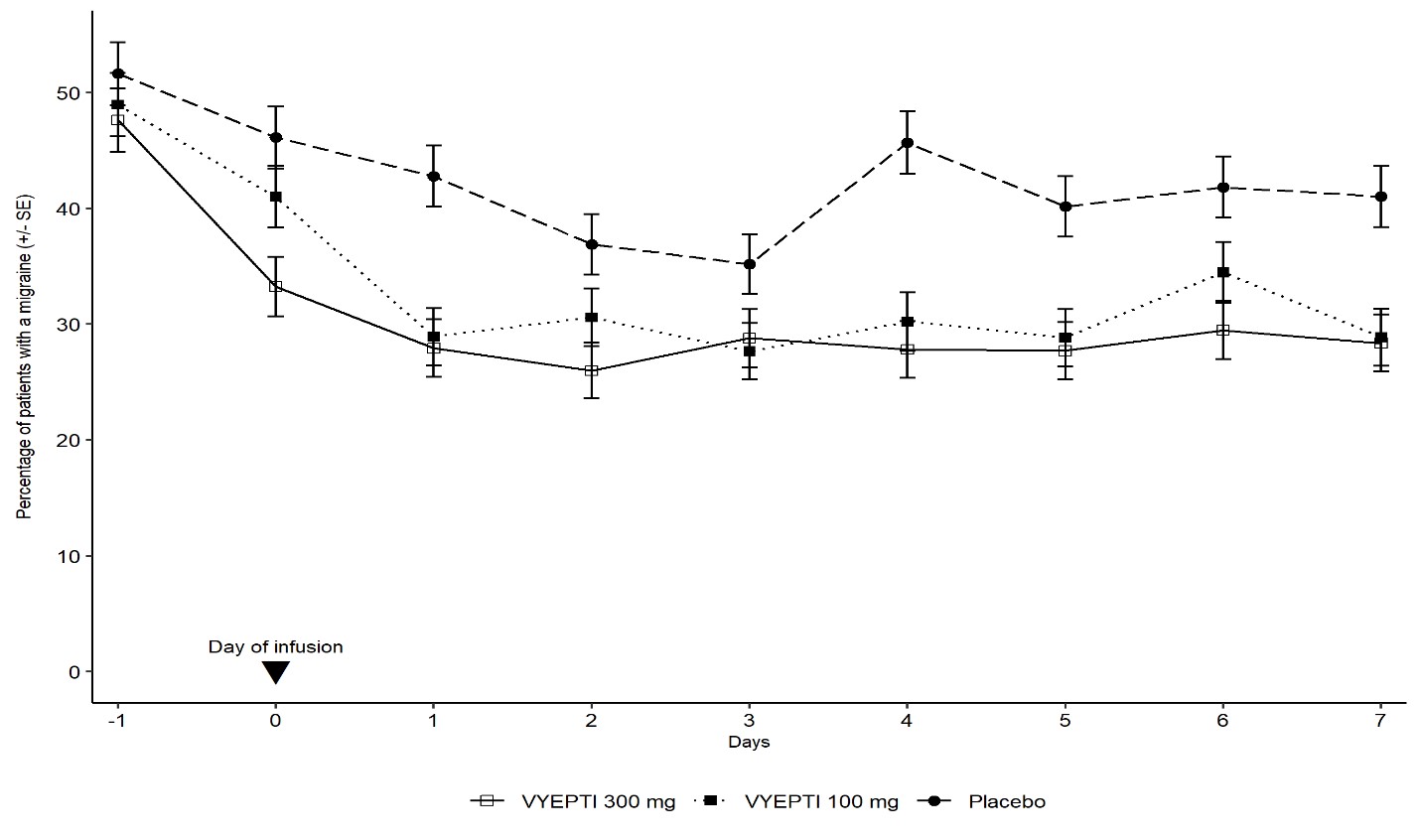
In clinical trials, patients receiving Vyepti experienced significant reductions in monthly migraine days, often within the first month after treatment (FDA, 2020). Many participants reported improved productivity, less disability, and better overall quality of life.
Unlike acute migraine medications that provide short-term relief, Vyepti works on an ongoing basis to reduce the frequency, duration, and intensity of migraines. This can be life-changing for those who experience chronic migraines, defined as 15 or more headache days per month.
How does Vyepti work?
Migraines are thought to involve complex interactions between nerves and blood vessels in the brain, with a key role played by a molecule called calcitonin gene-related peptide (CGRP). CGRP levels rise during migraine attacks, causing blood vessels to dilate and triggering pain pathways.
Vyepti is a monoclonal antibody that binds to CGRP, blocking its activity before it can initiate the chain reaction that leads to migraine symptoms. By neutralizing CGRP, Vyepti helps prevent the inflammatory and vascular changes that drive migraine pain.
Clinically, this targeted approach matters because it addresses the root cause of migraine attacks rather than just treating symptoms. Patients who respond well often experience fewer severe migraines and require less rescue medication over time (Mayo Clinic, 2023).
Vyepti side effects
Vyepti is generally well tolerated, with most side effects being mild to moderate.
Common side effects include:
- Runny nose or sore throat (nasopharyngitis)
- Fatigue
- Mild allergic reactions such as itching or rash
Serious but uncommon side effects:
- Hypersensitivity reactions, which may include swelling, flushing, or shortness of breath
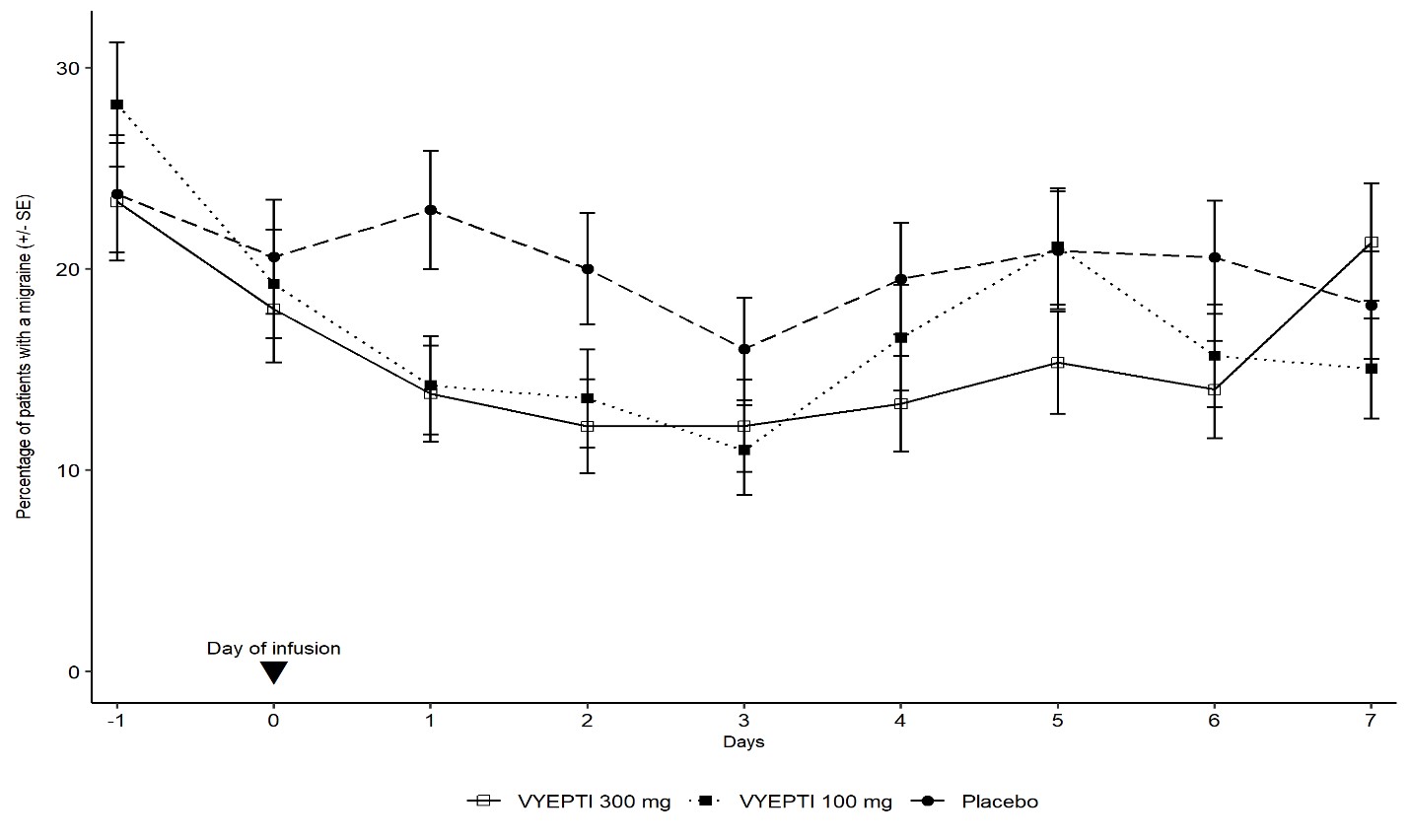
- Infusion-related reactions, typically occurring during or shortly after the IV infusion
Most reactions resolve without intervention, but patients should notify their healthcare team if they experience persistent or severe symptoms.
Those with a known allergy to eptinezumab or its components should not receive Vyepti. Pregnant or breastfeeding women should discuss potential risks and benefits with their doctor, as safety data in these populations are still limited (MedlinePlus, 2024).
Vyepti dosage
Vyepti is given as an intravenous infusion every 12 weeks in a clinical setting, typically a doctor’s office or infusion center. The infusion usually takes about 30 minutes.
One of the key advantages of Vyepti is its quarterly dosing schedule, which minimizes the need for frequent treatments. Because it is administered under medical supervision, healthcare professionals can monitor any infusion-related reactions during and after the procedure.
Routine laboratory testing is not usually required, but doctors may evaluate:
- Treatment response, such as changes in migraine frequency
- Adverse reactions or allergic symptoms over time
Patients are encouraged to keep a migraine diary to track the frequency and intensity of their headaches, which helps their provider assess how well the therapy works.
Does Vyepti have a generic version?
As of 2025, there is no generic version of Vyepti available. It is currently only offered as the brand-name product manufactured by Lundbeck Pharmaceuticals.
Because Vyepti is a biologic drug, a large, complex protein molecule produced through living cells, creating a generic equivalent is not straightforward. Instead, similar versions called biosimilars may be developed in the future once Vyepti’s exclusivity period expires.
Patients concerned about cost should speak with their healthcare provider or pharmacist about insurance coverage and manufacturer assistance programs, as many companies offer financial support for eligible individuals.
Conclusion
Vyepti (eptinezumab-jjmr) represents a major advancement in migraine prevention. By targeting CGRP, the key molecule involved in migraine activation, it helps reduce the frequency of attacks and gives patients a chance to live more freely and confidently.
While not every patient responds the same way, many report meaningful improvements in their ability to function, sleep, and engage in daily activities. The quarterly infusion schedule also makes adherence easier and provides sustained migraine protection between treatments.
When prescribed and monitored by a qualified healthcare provider, Vyepti is a safe and effective preventive option for adults with frequent or disabling migraines. Patients considering this therapy should discuss whether it fits their health history, lifestyle, and long-term goals for migraine management.
References
- U.S. Food and Drug Administration (FDA). Vyepti (Eptinezumab-jjmr) Prescribing Information, 2020. https://www.fda.gov/
- Mayo Clinic. Eptinezumab (Intravenous Route) Description and Side Effects, 2023. https://www.mayoclinic.org/
- MedlinePlus. Eptinezumab Injection – Drug Information, National Library of Medicine, 2024. https://medlineplus.gov/
Approved To Treat
Top Global Experts
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
- Injection: 100 mg/mL in a single-dose vial
- Hypersensitivity Reactions
- Hypertension
- Raynaud’s Phenomenon