Generic Name
Glargine
Brand Names
Toujeo, Glargine U-300, Lantus Solostar, Lantus, Glargine Solostar, Rezvoglar, Semglee, Basaglar
FDA approval date: September 23, 2009
Classification: Insulin Analog
Form: Injection
What is Toujeo (Glargine)?
Insulin glargine is indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus. Insulin glargine is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus. Limitations of Use Not recommended for treating diabetic ketoacidosis. Limitations of Use Insulin glargine is not recommended for the treatment of diabetic ketoacidosis.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
TOUJEO (insulin glargine)
1INDICATIONS AND USAGE
TOUJEO is indicated to improve glycemic control in adults and pediatric patients 6 years of age and older with diabetes mellitus.
2DOSAGE FORMS AND STRENGTHS
Injection: 300 units/mL (U-300) of insulin glargine available as a clear, colorless, solution in:
- 1.5 mL TOUJEO SoloStar single-patient-use prefilled pen (450 units per 1.5 mL pen)
- 3 mL TOUJEO Max SoloStar single-patient-use prefilled pen (900 units per 3 mL pen)
3CONTRAINDICATIONS
TOUJEO is contraindicated:
- During episodes of hypoglycemia
- In patients with hypersensitivity to insulin glargine or any excipients in TOUJEO
4ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere:
- Hypoglycemia
- Hypoglycemia due to medication errors
- Hypersensitivity reactions
- Hypokalemia
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates actually observed in clinical practice.
The data in Table 1 reflect the exposure of 304 patients with type 1 diabetes to TOUJEO with mean exposure duration of 23 weeks. The type 1 diabetes population had the following characteristics: Mean age was 46 years and mean duration of diabetes was 21 years. Fifty-five percent were male, 86% were Caucasian, 5% were Black or African American, and 5% were Hispanic. At baseline, the mean eGFR was 82 mL/min/1.73 m
The data in Table 2 reflect the exposure of 1242 patients with type 2 diabetes to TOUJEO with mean exposure duration of 25 weeks. The type 2 diabetes population had the following characteristics: Mean age was 59 years and mean duration of diabetes was 13 years. Fifty-three percent were male, 88% were Caucasian, 7% were Black or African American, and 17% were Hispanic. At baseline, mean eGFR was 79 mL/min/1.73 m
TOUJEO was studied in 233 pediatric patients (6–17 years of age) with type 1 diabetes for a mean duration of 26 weeks
Common adverse reactions (occurring ≥5%) in TOUJEO-treated subjects during clinical trials in adult patients with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in Table 1 and Table 2, respectively. Common adverse reactions for TOUJEO-treated pediatric subjects with type 1 diabetes mellitus were similar to the adverse reactions listed in Table 1. Hypoglycemia is discussed in a dedicated subsection below.
4.2Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity.
In a 6-month study of type 1 diabetes patients, 79% of patients who received TOUJEO once daily were positive for anti-insulin antibodies (AIA) at least once during the study, including 62% that were positive at baseline and 44% of patients who developed antidrug antibody (i.e., anti-insulin glargine antibody [ADA]) during the study. Eighty percent of the AIA-positive patients on TOUJEO with antibody test at baseline remained AIA positive at month 6.
In two 6-month studies in type 2 diabetes patients, 25% of patients who received TOUJEO once daily were positive for AIA at least once during the study, including 42% who were positive at baseline and 20% of patients who developed ADA during the study. Ninety percent of the AIA-positive patients on TOUJEO with antibody test at baseline, remained AIA positive at month 6.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to TOUJEO with the incidence of antibodies in other studies or to other products may be misleading.
4.3Postmarketing Experience
The following additional adverse reactions have been identified during postapproval use of TOUJEO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
5DRUG INTERACTIONS
Table 3 includes clinically significant drug interactions with TOUJEO.
6OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia
7DESCRIPTION
Insulin glargine is a long-acting human insulin analog produced by recombinant DNA technology utilizing a nonpathogenic laboratory strain of
TOUJEO (insulin glargine) injection is a sterile, clear and colorless solution for subcutaneous injection. Each mL of TOUJEO contains 300 units of insulin glargine dissolved in a clear aqueous fluid.
The 1.5 mL TOUJEO SoloStar prefilled pen presentation contains the following inactive ingredients per mL: glycerin (20 mg), metacresol (2.7 mg), zinc (90 mcg), and Water for Injection, USP.
The 3 mL TOUJEO Max SoloStar prefilled pen presentation contains the following inactive ingredients per mL: glycerin (20 mg), metacresol (2.7 mg), zinc (90 mcg), and Water for Injection, USP.
The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. TOUJEO has a pH of approximately 4.
8HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-2193
NDC: 50090-2193-0 1.5 mL in a SYRINGE / 3 in a CARTON
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). There are separate Instructions for Use for TOUJEO SoloStar and TOUJEO Max SoloStar.
10Instructions for Use
TOUJEO
Read this first
Do not share your TOUJEO SoloStar pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
TOUJEO contains 300 units/mL of insulin glargine
- Do not re-use needles. If you do, you might not get your dose (underdosing) or get too much (overdosing) as the needle could block.
- Do not use a syringe to remove insulin from your pen. If you do, you will get too much insulin. The scale on most syringes is made for U-100 (non-concentrated) insulin only.
- The dose selector of your TOUJEO SoloStar pen dials by 1 unit.
People who are blind or have vision problems should not use the TOUJEO SoloStar pen without help from a person trained to use the TOUJEO SoloStar pen.
Important information
- Do not use your pen if it is damaged or if you are not sure that it is working properly.
- Always perform a safety test (see
- Always carry a spare pen and spare needles in case they are lost or stop working.
- Change (rotate) your injection sites within the area you choose for each dose (see
Learn to inject
- Talk with your healthcare provider about how to inject, before using your pen.
- Read all of these instructions before using your pen. If you do not follow all of these instructions, you may get too much or too little insulin.
Need help?
If you have any questions about your pen or about diabetes, ask your healthcare provider, go to
Extra items you will need:
- a new sterile needle (not included with the pen) (see
- an alcohol swab.
- a puncture-resistant container for used needles and pens (see
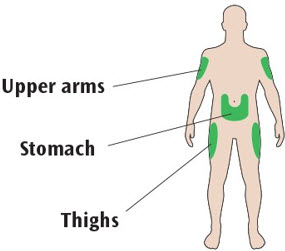
Places to inject
- Inject your insulin exactly as your healthcare provider has shown you.
- Inject your insulin under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting pits or thickening of the skin (lipodystrophy) and lumps in the skin (localized cutaneous amyloidosis) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
Get to know your pen
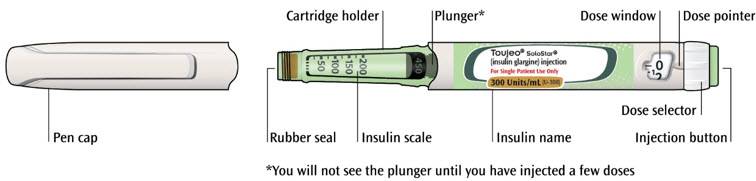
Step 1: Check your pen
Take a new pen out of the refrigerator at least
- Check the name and expiration date on the label of your pen.
- Make sure you have the correct insulin.
- Do not use your pen after the expiration date printed on the label.
- Make sure you have the correct insulin.
- Pull off the pen cap.
- Check that the insulin is clear.
- Do not use the pen if the insulin looks cloudy, colored or contains particles.
- Do not use the pen if the insulin looks cloudy, colored or contains particles.
- Wipe the rubber seal with an alcohol swab.
If you have other injector pens
- Making sure you have the correct medicine is especially important if you have other injector pens.
Step 2: Attach a new needle
- Do not re-use needles. Always use a new sterile needle for each injection. This helps stop blocked needles, contamination and infection.
- Always use needles
- Take a new needle and peel off the protective seal.
- Keep the needle straight and screw it onto the pen until fixed. Do not over-tighten.
- Pull off the outer needle cap. Keep this for later.
- Pull off the inner needle cap and throw away.
Handling needles
- Be careful when you are handling needles to help prevent accidental needle-stick injury. You may give other people a serious infection, or get a serious infection from them.
Step 3: Do a safety test
Always do a safety test before each injection to:
- check your pen and the needle to make sure they are working properly.
- make sure that you get the correct insulin dose.
If the pen is new, you must perform safety tests before you use the pen for the first time until you see insulin coming out of the needle tip. If you see insulin coming out of the needle tip, the pen is ready to use. If you do not see insulin coming out before taking your dose, you could get an underdose or no insulin at all. This could cause high blood sugar.
- Select 3 units by turning the dose selector until the dose pointer is at the mark between 2 and 4.
- Press the injection button all the way in.
- When insulin comes out of the needle tip, your pen is working correctly.
If no insulin appears:
- You may need to repeat this step up to 3 times before seeing insulin.
- If no insulin comes out after the third time, the needle may be blocked. If this happens:
- Do not use your pen if there is still no insulin coming out of the needle tip. Use a new pen.
- Do not use a syringe to remove insulin from your pen.
If you see air bubbles
- You may see air bubbles in the insulin. This is normal, they will not harm you.
Step 4: Select the dose
- Do not select a dose or press the injection button without a needle attached. This may damage your pen.
- TOUJEO SoloStar is made to deliver the number of insulin units that your healthcare provider prescribed. You
- The dose selector of your TOUJEO SoloStar pen dials by 1 unit.
- Make sure a needle is attached and the dose is set to "0."
- Turn the dose selector until the dose pointer lines up with your dose.
- Set the dose by turning the dose selector to a line in the dose window. Each line equals 1 unit.
- The dose selector clicks as you turn it.
- Always check the number in the dose window to make sure you dialed the correct dose.
- Do not dial your dose by counting the clicks. You may dial the wrong dose. This may lead to you getting too much insulin or not enough insulin.
- If you turn past your dose, you can turn back down.
- If there are not enough units left in your pen for your dose, the dose selector will stop at the number of units left.
- If you cannot select your full prescribed dose, split the dose into
How to read the dose window
The dose selector dials by 1 unit.
Even numbers are shown in line with the dose pointer:

30 units selected
Odd numbers are shown as a line between even numbers:

29 units selected
Units of insulin in your pen
- Your pen contains a total of
- You can see roughly how many units of insulin are left by looking at where the plunger is on the insulin scale.
Step 5: Inject your dose
If you find it hard to press the injection button in,
- Choose a place to inject as shown in the picture labeled "Places to inject."
- The site you choose for the injection should be clean and dry.
- If your skin is dirty, clean it as instructed by your healthcare provider.
- Push the needle into your skin as shown by your healthcare provider.
- Do not touch the injection button yet.
- Do not touch the injection button yet.
- Place your thumb on the injection button. Then press all the way in and hold.
- Do not press at an angle. Your thumb could block the dose selector from turning.
- Do not press at an angle. Your thumb could block the dose selector from turning.
- Keep the injection button held in and when you see "0" in the dose window, slowly count to 5.
- This will make sure you get your full dose.
- After holding and slowly counting to 5, release the injection button. Then remove the needle from your skin.
If you find it hard to press the injection button in:
- Change the needle (see
- If you still find it hard to press in, get a new pen.
- Do not use a syringe to remove insulin from your pen.
Step 6: Remove the needle
- Take care when handling needles to prevent needle injury and cross-infection.
- Do not put the inner needle cap back on.
- Grip the widest part of the outer needle cap. Keep the needle straight and guide it into the outer needle cap.
- The needle can puncture the cap if it is recapped at an angle.
- Grip and squeeze the widest part of the outer needle cap. Turn your pen several times with your other hand to remove the needle.
- Try again if the needle does not come off the first time.
- Throw away the used needle in a puncture-resistant container (see " at the end of this Instructions for Use).
- Put the pen cap back on.
- Do not put the pen back in the refrigerator.
- Do not put the pen back in the refrigerator.
Use by
- Only use your pen for up to
How to store your pen
Before first use
- Keep new pens in the refrigerator between
- Do not freeze. Throw away your pen if it has been frozen (See "Throwing your pen away").
After first use
- Keep your pen at room temperature
- Protect your pen from direct heat and light.
- Do not put your pen back in the refrigerator.
- Do not store your pen with the needle attached.
- Store your pen with the pen cap on.
- Keep TOUJEO SoloStar pens and needles out of the reach of children.
How to care for your pen
Handle your pen with care
- Do not drop your pen or knock it against hard surfaces.
- If you think that your pen may be damaged,
Protect your pen from dust and dirt
- You can clean the outside of your pen by wiping it with a damp cloth (water only).
Throwing your pen away
- The used TOUJEO SoloStar pen may be thrown away in your household trash after you have removed the needle.
- Put the used needle in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Manufactured by:
©2022 sanofi-aventis U.S. LLC
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: August 2022
11Instructions for Use
TOUJEO
Read this first
Do not share your TOUJEO Max SoloStar pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
TOUJEO contains 300 units/mL of insulin glargine
- Do not re-use needles. If you do, you might not get your dose (underdosing) or get too much (overdosing) as the needle could block.
- Do not use a syringe to remove insulin from your pen. If you do, you will get too much insulin. The scale on most syringes is made for U-100 (non-concentrated) insulin only.
- The dose selector of your TOUJEO Max SoloStar pen dials by
People who are blind or have vision problems should not use the TOUJEO Max SoloStar pen without help from a person trained to use the TOUJEO Max SoloStar pen.
Important information
- Do not use your pen if it is damaged or if you are not sure that it is working properly.
- Always perform a safety test (see
- Always carry a spare pen and spare needles in case they are lost or stop working.
- Change (rotate) your injection sites within the area you choose for each dose (see
Learn to inject
- Talk with your healthcare provider about how to inject, before using your pen.
- Read all of these instructions before using your pen. If you do not follow all of these instructions, you may get too much or too little insulin.
Need help?
If you have any questions about your pen or about diabetes, ask your healthcare provider, go to
Extra items you will need:
- a new sterile needle (not included with the pen) (see
- an alcohol swab.
- a puncture-resistant container for used needles and pens (see
Places to inject
- Inject your insulin exactly as your healthcare provider has shown you.
- Inject your insulin under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting pits or thickening of the skin (lipodystrophy) and lumps in the skin (localized cutaneous amyloidosis) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
Get to know your pen
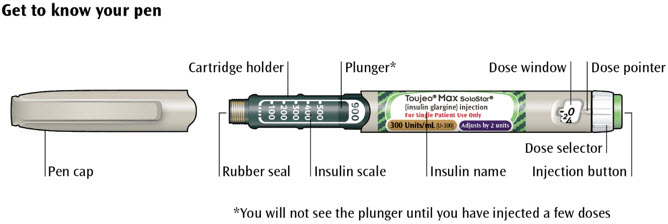
Step 1: Check your pen
Take a new pen out of the refrigerator at least
- Check the name and expiration date on the label of your pen.
- Make sure you have the correct insulin.
- Do not use your pen after the expiration date printed on the label.
- Make sure you have the correct insulin.
- Pull off the pen cap.
- Check that the insulin is clear.
- Do not use the pen if the insulin looks cloudy, colored or contains particles.
- Do not use the pen if the insulin looks cloudy, colored or contains particles.
- Wipe the rubber seal with an alcohol swab.
If you have other injector pens
- Making sure you have the correct medicine is especially important if you have other injector pens.
Step 2: Attach a new needle
- Do not re-use needles. Always use a new sterile needle for each injection. This helps stop blocked needles, contamination and infection.
- Always use needles
- Take a new needle and peel off the protective seal.
- Keep the needle straight and screw it onto the pen until fixed. Do not over-tighten.
- Pull off the outer needle cap. Keep this for later.
- Pull off the inner needle cap and throw away.
Handling needles
- Be careful when you are handling needles to help prevent accidental needle-stick injury. You may give other people a serious infection, or get a serious infection from them.
Step 3: Do a safety test
Always do a safety test before each injection to:
- check your pen and the needle to make sure they are working properly.
- make sure that you get the correct insulin dose.
If the pen is new, you must perform safety tests before you use the pen for the first time until you see insulin coming out of the needle tip. If you see insulin coming out of the needle tip, the pen is ready to use. If you do not see insulin coming out before taking your dose, you could get an underdose or no insulin at all. This could cause high blood sugar.
- Select 4 units by turning the dose selector until the dose pointer is at the 4 mark.
- Press the injection button all the way in.
- When insulin comes out of the needle tip, your pen is working correctly.
If no insulin appears:
- You may need to repeat this step up to 6 times before seeing insulin.
- If no insulin comes out after the sixth time, the needle may be blocked. If this happens:
- Do not use your pen if there is still no insulin coming out of the needle tip. Use a new pen.
- Do not use a syringe to remove insulin from your pen.
If you see air bubbles
- You may see air bubbles in the insulin. This is normal, they will not harm you.
Step 4: Select the dose
- Do not select a dose or press the injection button without a needle attached. This may damage your pen.
- TOUJEO Max SoloStar is made to deliver the number of insulin units that your healthcare provider prescribed. You
- The dose selector of your TOUJEO Max SoloStar pen dials by 2 units and can only dial even doses of insulin.
- Make sure a needle is attached and the dose is set to "0."
- Turn the dose selector until the dose pointer lines up with your dose.
- Set the dose by turning the dose selector to a line in the dose window. Each line equals 2 units.
- The dose selector clicks as you turn it.
- Always check the number in the dose window to make sure you dialed the correct dose.
- Do not dial your dose by counting the clicks. You may dial the wrong dose. This may lead to you getting too much insulin or not enough insulin.
- If you turn past your dose, you can turn back down.
- If there are not enough units left in your pen for your dose, the dose selector will stop at the number of units left.
- If you cannot select your full prescribed dose, split the dose into
How to read the dose window
The dose selector dials by 2 units.
Each line in the dose window is an even number.

60 units selected

58 units selected
Units of insulin in your pen
- Your pen contains a total of
- You can see roughly how many units of insulin are left by looking at where the plunger is on the insulin scale.
Step 5: Inject your dose
If you find it hard to press the injection button in,
- Choose a place to inject as shown in the picture labeled "Places to inject."
- The site you choose for the injection should be clean and dry.
- If your skin is dirty, clean it as instructed by your healthcare provider.
- Push the needle into your skin as shown by your healthcare provider.
- Do not touch the injection button yet.
- Do not touch the injection button yet.
- Place your thumb on the injection button. Then press all the way in and hold.
- Do not press at an angle. Your thumb could block the dose selector from turning.
- Do not press at an angle. Your thumb could block the dose selector from turning.
- Keep the injection button held in and when you see "0" in the dose window, slowly count to 5.
- This will make sure you get your full dose.
- After holding and slowly counting to 5, release the injection button. Then remove the needle from your skin.
If you find it hard to press the injection button in:
- Change the needle (see
- If you still find it hard to press in, get a new pen.
- Do not use a syringe to remove insulin from your pen.
Step 6: Remove the needle
- Take care when handling needles to prevent needle injury and cross-infection.
- Do not put the inner needle cap back on.
- Grip the widest part of the outer needle cap. Keep the needle straight and guide it into the outer needle cap.
- The needle can puncture the cap if it is recapped at an angle.
- Grip and squeeze the widest part of the outer needle cap. Turn your pen several times with your other hand to remove the needle.
- Try again if the needle does not come off the first time.
- Throw away the used needle in a puncture-resistant container (see " at the end of this Instructions for Use).
- Put the pen cap back on.
- Do not put the pen back in the refrigerator.
- Do not put the pen back in the refrigerator.
Use by
- Only use your pen for up to
How to store your pen
Before first use
- Keep new pens in the refrigerator between
- Do not freeze. Throw away your pen if it has been frozen (see ").
After first use
- Keep your pen at room temperature
- Protect your pen from direct heat and light.
- Do not put your pen back in the refrigerator.
- Do not store your pen with the needle attached.
- Store your pen with the pen cap on.
- Keep TOUJEO Max SoloStar pens and needles out of the reach of children.
How to care for your pen
Handle your pen with care
- Do not drop your pen or knock it against hard surfaces.
- If you think that your pen may be damaged,
Protect your pen from dust and dirt
- You can clean the outside of your pen by wiping it with a damp cloth (water only).
Throwing your pen away
- The used TOUJEO Max SoloStar pen may be thrown away in your household trash after you have removed the needle.
- Put the used needle in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Manufactured by:
©2022 sanofi-aventis U.S. LLC
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: August 2022
12insulin glargine
