Brand Name
Xenazine
Generic Name
Tetrabenazine
View Brand Information FDA approval date: November 24, 2008
Classification: Vesicular Monoamine Transporter 2 Inhibitor
Form: Tablet
What is Xenazine (Tetrabenazine)?
Tetrabenazine tablets are indicated for the treatment of chorea associated with Huntington’s disease. Tetrabenazine tablets are a vesicular monoamine transporter 2 inhibitor indicated for the treatment of chorea associated with Huntington’s disease.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Xenazine (tetrabenazine)
WARNING: DEPRESSION AND SUICIDALITY
XENAZINE can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Anyone considering the use of XENAZINE must balance the risks of depression and suicidality with the clinical need for control of chorea. Close observation of patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior should accompany therapy. Patients, their caregivers, and families should be informed of the risk of depression and suicidality and should be instructed to report behaviors of concern promptly to the treating physician.
Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in Huntington's disease. XENAZINE is contraindicated in patients who are actively suicidal, and in patients with untreated or inadequately treated depression
1INDICATIONS AND USAGE
XENAZINE is indicated for the treatment of chorea associated with Huntington’s disease.
2DOSAGE FORMS AND STRENGTHS
XENAZINE tablets are available in the following strengths and packages:
The 12.5 mg XENAZINE tablets are white, cylindrical, biplanar tablets with beveled edges, non-scored, embossed on one side with “CL” and “12.5.”
The 25 mg XENAZINE tablets are yellowish-buff, cylindrical, biplanar tablets with beveled edges, scored, embossed on one side with “CL” and “25.”
3CONTRAINDICATIONS
XENAZINE is contraindicated in patients:
- Who are actively suicidal, or in patients with untreated or inadequately treated depression
- With hepatic impairment
- Taking monoamine oxidase inhibitors (MAOIs). XENAZINE should not be used in combination with an MAOI, or within a minimum of 14 days of discontinuing therapy with an MAOI
- Taking reserpine. At least 20 days should elapse after stopping reserpine before starting XENAZINE
- Taking deutetrabenazine or valbenazine
4ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Depression and Suicidality
- Neuroleptic Malignant Syndrome (NMS)
- Akathisia, Restlessness, and Agitation
- Parkinsonism
- Sedation and Somnolence
- QTc Prolongation
- Hypotension and Orthostatic Hypotension
- Hyperprolactinemia
- Binding to Melanin-Containing Tissues
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During its development, XENAZINE was administered to 773 unique subjects and patients. The conditions and duration of exposure to XENAZINE varied greatly, and included single-dose and multiple-dose clinical pharmacology studies in healthy volunteers (n=259) and open-label (n=529) and double-blind studies (n=84) in patients.
In a randomized, 12-week, placebo-controlled clinical trial of HD patients, adverse reactions were more common in the XENAZINE group than in the placebo group. Forty-nine of 54 (91%) patients who received XENAZINE experienced one or more adverse reactions at any time during the study. The most common adverse reactions (over 10%, and at least 5% greater than placebo) were sedation/somnolence, fatigue, insomnia, depression, akathisia, anxiety/anxiety aggravated, and nausea.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of XENAZINE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous system disorders: tremor
Psychiatric disorders: confusion, worsening aggression
Respiratory, thoracic and mediastinal disorders: pneumonia
Skin and subcutaneous tissue disorders: hyperhidrosis, skin rash
5OVERDOSAGE
Three episodes of overdose occurred in the open-label trials performed in support of registration. Eight cases of overdose with XENAZINE have been reported in the literature. The dose of XENAZINE in these patients ranged from 100 mg to 1 g. Adverse reactions associated with XENAZINE overdose include acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor.
Treatment should consist of those general measures employed in the management of overdosage with any CNS-active drug. General supportive and symptomatic measures are recommended. Cardiac rhythm and vital signs should be monitored. In managing overdosage, the possibility of multiple drug involvement should always be considered. The physician should consider contacting a poison control center on the treatment of any overdose.
6DESCRIPTION
XENAZINE (tetrabenazine) is a monoamine depletor for oral administration. The molecular weight of tetrabenazine is 317.43; the pKa is 6.51. Tetrabenazine is a hexahydro-dimethoxy-benzoquinolizine derivative and has the following chemical name: cis rac –1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-one.
The empirical formula C
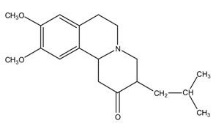
Tetrabenazine is a white to slightly yellow crystalline powder that is sparingly soluble in water and soluble in ethanol.
Each XENAZINE (tetrabenazine) tablet contains either 12.5 or 25 mg of tetrabenazine as the active ingredient.
XENAZINE (tetrabenazine) tablets contain tetrabenazine as the active ingredient and the following inactive ingredients: lactose, magnesium stearate, maize starch, and talc. The 25 mg strength tablet also contains yellow iron oxide as an inactive ingredient.
XENAZINE (tetrabenazine) tablets are supplied as a yellowish-buff, scored tablet containing 25 mg of tetrabenazine or as a white, non-scored tablet containing 12.5 mg of tetrabenazine.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risk of Suicidality
Inform patients and their families that XENAZINE may increase the risk of suicidal thinking and behaviors. Counsel patients and their families to remain alert to the emergence of suicidal ideation and to report it immediately to the patient’s physician
Risk of Depression
Inform patients and their families that XENAZINE may cause depression or may worsen pre-existing depression. Encourage patients and their families to be alert to the emergence of sadness, worsening of depression, withdrawal, insomnia, irritability, hostility (aggressiveness), akathisia (psychomotor restlessness), anxiety, agitation, or panic attacks and to report such symptoms promptly to the patient’s physician
Dosing of XENAZINE
Inform patients and their families that the dose of XENAZINE will be increased slowly to the dose that is best for each patient. Sedation, akathisia, parkinsonism, depression, and difficulty swallowing may occur. Such symptoms should be promptly reported to the physician, and the XENAZINE dose may need to be reduced or discontinued
Risk of Sedation and Somnolence
Inform patients that XENAZINE may induce sedation and somnolence and may impair the ability to perform tasks that require complex motor and mental skills. Advise patients that until they learn how they respond to XENAZINE, they should be careful doing activities that require them to be alert, such as driving a car or operating machinery
Interaction with Alcohol
Advise patients and their families that alcohol may potentiate the sedation induced by XENAZINE
Usage in Pregnancy
Advise patients and their families to notify the physician if the patient becomes pregnant or intends to become pregnant during XENAZINE therapy, or is breastfeeding or intending to breastfeed an infant during therapy
Manufactured by:
Recipharm Fontaine SAS
Rue des Prés Potets
21121 Fontaine-lés-Dijon, France
Recipharm Fontaine SAS
Rue des Prés Potets
21121 Fontaine-lés-Dijon, France
Manufactured for:
Lundbeck
Deerfield, IL 60015 USA
Lundbeck
Deerfield, IL 60015 USA
Xenazine is a trademark of Bausch Health Companies Inc. or its affiliates.
All other product/brand names and/or logos are trademarks of the respective owners.
© 2020 Bausch Health Companies Inc. or its affiliates
9465504
8MEDICATION GUIDE
XENAZINE
(tetrabenazine) Tablets
(tetrabenazine) Tablets
Read the Medication Guide that comes with XENAZINE before you start taking it and each time you refill the prescription. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. You should share this information with your family members and caregivers.
What is the most important information I should know about XENAZINE?
- XENAZINE can cause serious side effects, including:
- depression
- suicidal thoughts
- suicidal actions
- You should not start taking XENAZINE if you are depressed (have untreated depression or depression that is not well controlled by medicine)
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts or feelings. This is especially important when XENAZINE is started and when the dose is changed.
Call the doctor right away if you become depressed or have any of the following symptoms, especially if they are new, worse, or worry you:
- feel sad or have crying spells
- lose interest in seeing your friends or doing things you used to enjoy
- sleep a lot
- feel unimportant
- feel guilty
- feel hopeless or helpless
- more irritable, angry or aggressive than usual
- more or less hungry than usual or notice a big change in your body weight
- have trouble paying attention
- feel tired or sleepy all the time
- have thoughts about hurting yourself or ending your life
What is XENAZINE?
XENAZINE is a medicine that is used to treat the involuntary movements (chorea) of Huntington’s disease. XENAZINE does not cure the cause of the involuntary movements, and it does not treat other symptoms of Huntington’s disease, such as problems with thinking or emotions.
It is not known whether XENAZINE is safe and effective in children.
Who should not take XENAZINE?
Do not take XENAZINE if you:
- are depressed or have thoughts of suicide. See
- have liver problems.
- are taking a monoamine oxidase inhibitor (MAOI) medicine. Ask your doctor or pharmacist if you are not sure.
- are taking reserpine.
What should I tell my doctor before taking XENAZINE?
Tell your doctor about all your medical conditions, including if you:
- have emotional or mental problems (for example, depression, nervousness, anxiety, anger, agitation, psychosis, previous suicidal thoughts or suicide attempts).
- have liver disease.
- have any allergies. See the end of this Medication Guide for a complete list of the ingredients in XENAZINE.
- have breast cancer or a history of breast cancer.
- have heart disease that is not stable, have heart failure or recently had a heart attack.
- have an irregular heartbeat (cardiac arrhythmia).
- are pregnant or plan to become pregnant. It is not known if XENAZINE can harm your unborn baby.
- are breastfeeding. It is not known if XENAZINE passes into breast milk.
Tell your doctor about all the medicines you take, including prescription medicines and nonprescription medicines, vitamins and herbal products. Using XENAZINE with certain other medicines may cause serious side effects. Do not start
How should I take XENAZINE?
- XENAZINE is a tablet that you take by mouth.
- Take XENAZINE exactly as prescribed by your doctor.
- You may take XENAZINE with or without food.
- Your doctor will increase your dose of XENAZINE each week for several weeks, until you and your doctor find the best dose for you.
- If you stop taking XENAZINE or miss a dose, your involuntary movements may return or worsen in 12 to 18 hours after the last dose.
- Before starting XENAZINE, you should talk to your healthcare provider about what to do if you miss a dose. If you miss a dose and it is time for your next dose, do not double the dose.
- Tell your doctor if you stop taking XENAZINE for more than
- If your doctor thinks you need to take more than 50 mg of XENAZINE each day, you will need to have a blood test to see if it is safe for you.
What should I avoid while taking XENAZINE?
Sleepiness (sedation) is a common side effect of XENAZINE. While taking XENAZINE,
What are the possible side effects of XENAZINE?
XENAZINE can cause serious side effects, including:
- Depression, suicidal thoughts, or actions. See "What is the most important information I should know about XENAZINE?"
- Neuroleptic Malignant Syndrome (NMS). Call your doctor right away and go to the nearest emergency room if you develop these signs and symptoms that do not have another obvious cause:
- high fever
- stiff muscles
- problems thinking
- very fast or uneven heartbeat
- increased sweating
- Parkinsonism. Symptoms of Parkinsonism include: slight shaking, body stiffness, trouble moving or keeping your balance.
- Restlessness. You may get a condition where you feel a strong urge to move. This is called akathisia.
- Irregular heartbeat. XENAZINE increases your chance of having certain changes in the electrical activity in your heart which can be seen on an electrocardiogram (EKG). These changes can lead to a dangerous abnormal heartbeat. Taking XENAZINE with certain medicines may increase this chance.
- Dizziness due to blood pressure changes when you change position (orthostatic hypotension). Change positions slowly from lying down to sitting up and from sitting up to standing when taking XENAZINE. Tell your doctor right away if you get dizzy or faint while taking XENAZINE. Your doctor may need to watch your blood pressure closely.
Common side effects with XENAZINE include:
- sleepiness (sedation)
- trouble sleeping
- depression
- tiredness (fatigue)
- anxiety
- restlessness
- agitation
- nausea
Tell your doctor if you have any side effects. Do not stop taking XENAZINE without talking to your doctor first.
Call your doctor for medical advice about side effects. You may report side effects to the Food and Drug Administration (FDA) at 1-800-FDA-1088.
General information about XENAZINE
XENAZINE contains the active ingredient tetrabenazine. It also contains these inactive ingredients: lactose, maize starch, talc, and magnesium stearate. The 25 mg tablet, which is pale yellow, also contains yellow iron oxide.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use XENAZINE for a condition for which it was not prescribed. Do not give XENAZINE to other people, even if they have the same symptoms that you have. It may harm them. Keep XENAZINE out of the reach of children.
This Medication Guide summarizes the most important information about XENAZINE. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about XENAZINE that is written for healthcare professionals. You can also call Lundbeck’s XENAZINE Information Center at
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Recipharm Fontaine SAS
Rue des Prés Potets
21121 Fontaine-lés-Dijon, France
Recipharm Fontaine SAS
Rue des Prés Potets
21121 Fontaine-lés-Dijon, France
Manufactured for:
Lundbeck
Deerfield, IL 60015 USA
Lundbeck
Deerfield, IL 60015 USA
Xenazine is a trademark of Bausch Health Companies Inc. or its affiliates.
All other product/brand names and/or logos are trademarks of the respective owners.
© 2020 Bausch Health Companies Inc. or its affiliates
Revised 11/2019
9465504